Type-II diabetes preparation and preparation method thereof
A diabetes and preparation technology, applied in the field of type II diabetes preparations and their preparation, can solve the problems of ineffective control of postprandial blood sugar by insulin, cross allergy to sulfonamide drugs, damage to the liver and kidneys, etc. Utilization rate, the effect of reducing toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0055] The following clearly and completely describes the technical solutions in the embodiments of the present invention. Obviously, the described embodiments are part of the embodiments of the present invention, but not all of the embodiments. The detailed descriptions of the embodiments of the invention provided below are not intended to limit the scope of the claimed invention but represent only selected embodiments of the invention. Based on the embodiments of the present invention, all other embodiments obtained by persons of ordinary skill in the art without creative efforts fall within the protection scope of the present invention.
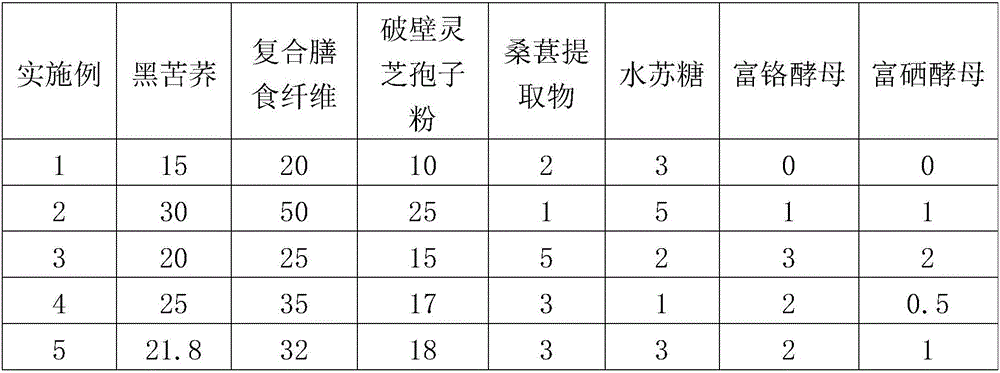
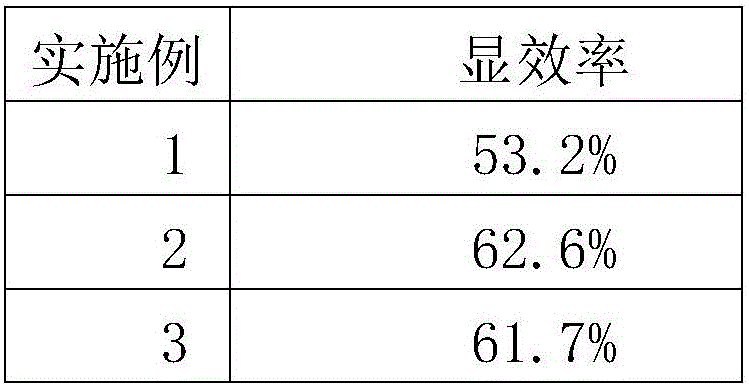
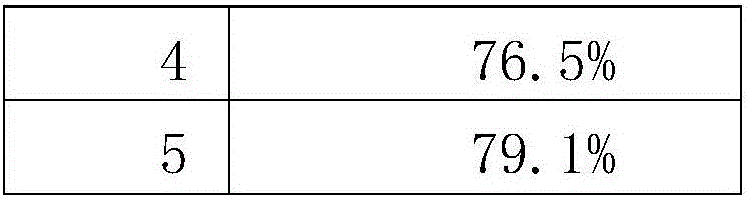
[0056] The present invention aims to provide a preparation for regulating type Ⅱ diabetes, which can effectively treat diabetes, comprehensively regulate metabolic disorders, enhance the immunity of the patient through conditioning and repairing the patient's body, restore the function of pancreatic islets, promote the secretion of insulin,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com