Synthesizing method of benzofuranone compound containing sulfonyl
A technology of benzofuranone and synthesis method, which is applied in directions such as organic chemistry and achieves the effects of mild conditions, safe operation and wide range of substrates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
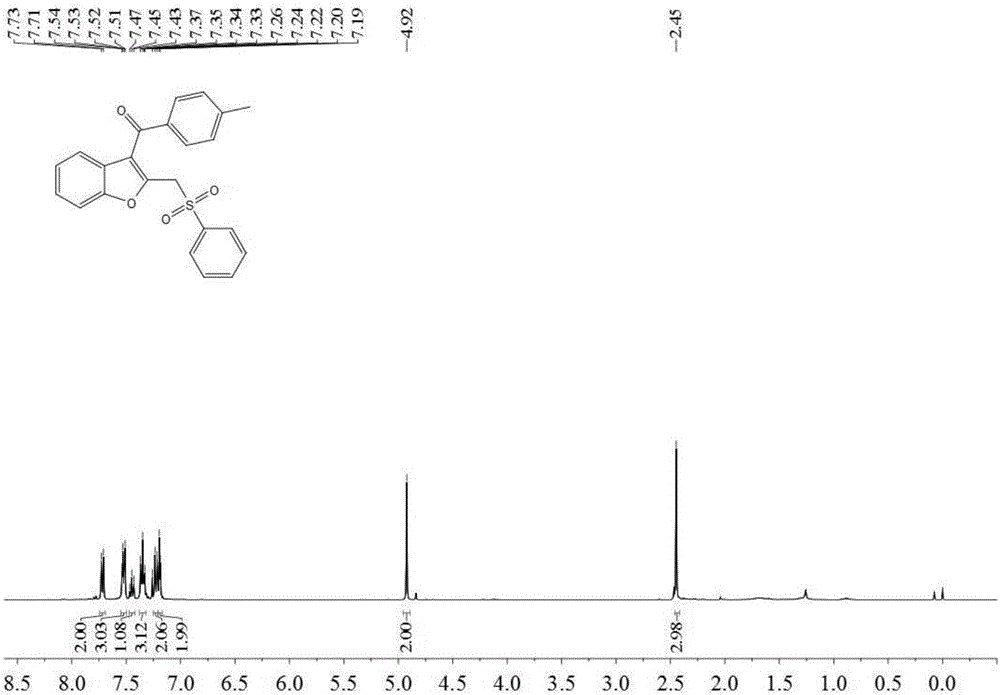
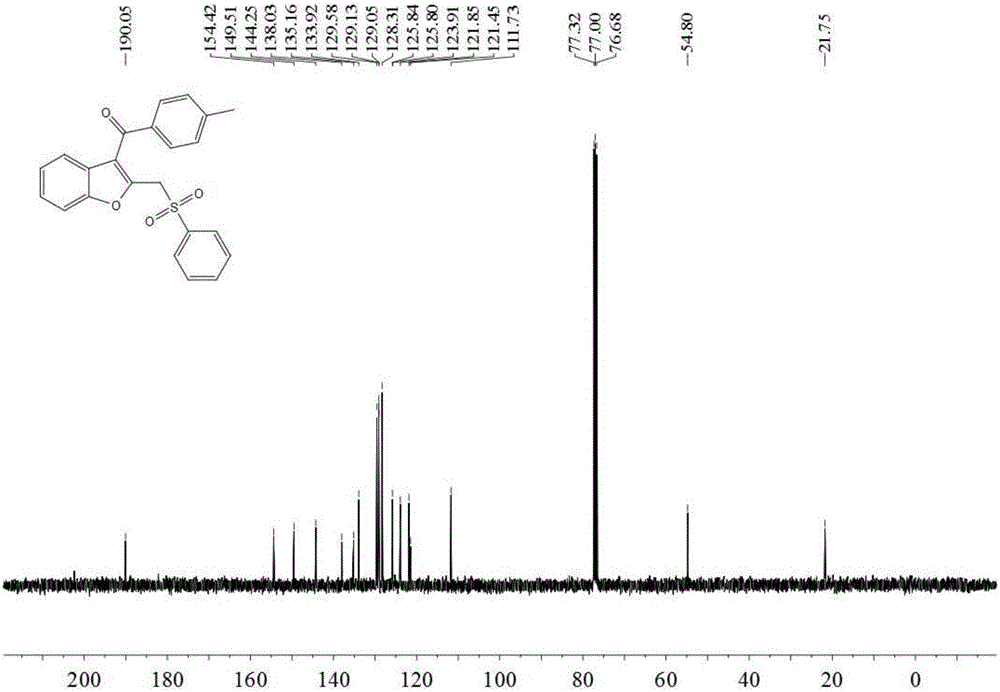
[0033] Add 0.3 mmol 2-(4-methylphenylethynyl) phenyl alkenyl ether (M.Hu, R-J.Song and J-H, Li, Angew.Chem.Int.Ed.2015,54,608– 612), 0.6 mmol of sodium benzene sulfinate, 0.06 mmol of silver nitrate, 0.6 mmol of potassium persulfate and 3 milliliters of acetonitrile, stirred and reacted at 80 degrees Celsius at 700 rpm for 4 hours, and stopped stirring. Add 4 mL of water, extract 3 times with ethyl acetate, combine the organic phases and use 0.5 g of anhydrous magnesium sulfate to dry, filter, evaporate the solvent under reduced pressure, and then separate and purify by column chromatography to obtain the target product. The eluent was petroleum ether: ethyl acetate mixed solvent with a volume ratio of 8:1, and the yield was 74%.
[0034] The hydrogen spectrogram and the carbon spectrogram of the product obtained in this embodiment are respectively as follows figure 1 and figure 2 shown; the structural characterization data are shown below:
[0035] 1 H NMR (400MHz, CDCl ...
Embodiment 2
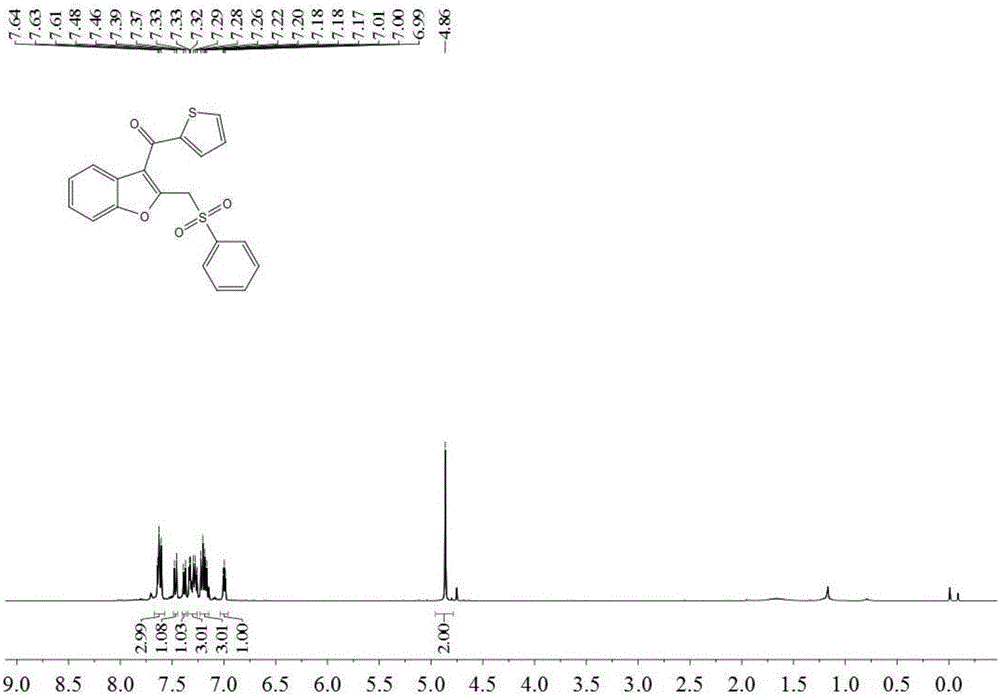
[0042]Add 0.3 mmol 2-thienylphenyl alkenyl ether (M.Hu, R-J. Song and J-H, Li, Angew. Chem. Int. Ed. 2015, 54, 608–612), 0.6 mmol phenylene oxide into the reaction tube Sodium sulfonate, 0.06 mmol of silver nitrate, 0.6 mmol of potassium persulfate and 3 ml of acetonitrile were stirred and reacted at 80 degrees Celsius at 700 rpm for 6 hours, and the stirring was stopped. Add 4 mL of water, extract 3 times with ethyl acetate, combine the organic phases and use 0.5 g of anhydrous magnesium sulfate to dry, filter, evaporate the solvent under reduced pressure, and then separate and purify by column chromatography to obtain the target product. The eluent was petroleum ether:ethyl acetate mixed solvent with a volume ratio of 5:1, and the yield was 62%.
[0043] The hydrogen spectrogram and the carbon spectrogram of the product obtained in this embodiment are respectively as follows image 3 and Figure 4 shown; the structural characterization data are shown below:
[0044] 1 H ...
Embodiment 3
[0051] Add 0.3 mmol 5-chloro-2-phenylethynylphenyl alkenyl ether (M.Hu, R-J. Songand J-H, Li, Angew. Chem. Int. Ed. 2015, 54, 608–612), 0.6 Millimole sodium benzenesulfinate, 0.06 mmol silver nitrate, 0.6 mmol potassium persulfate and 4 milliliters of acetonitrile were stirred and reacted at 80 degrees Celsius at 700 rpm for 4 hours, and the stirring was stopped. Add 4 mL of water, extract 3 times with ethyl acetate, combine the organic phases and use 0.5 g of anhydrous magnesium sulfate to dry, filter, evaporate the solvent under reduced pressure, and then separate and purify by column chromatography to obtain the target product. The eluent was petroleum ether:ethyl acetate mixed solvent with a volume ratio of 6:1, and the yield was 62%.
[0052] The hydrogen spectrogram and the carbon spectrogram of the product obtained in this embodiment are respectively as follows Figure 5 and Figure 6 shown; the structural characterization data are shown below:
[0053] 1 H NMR (400...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com