Method for treating avermectin oil
A technology of abamectin and processing method, applied in chemical instruments and methods, preparation of sugar derivatives, sugar derivatives, etc., can solve problems such as difficult handling and storage of abamectin ointment, and reduce unsafe factors , increase the content, improve the effect of benefit
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
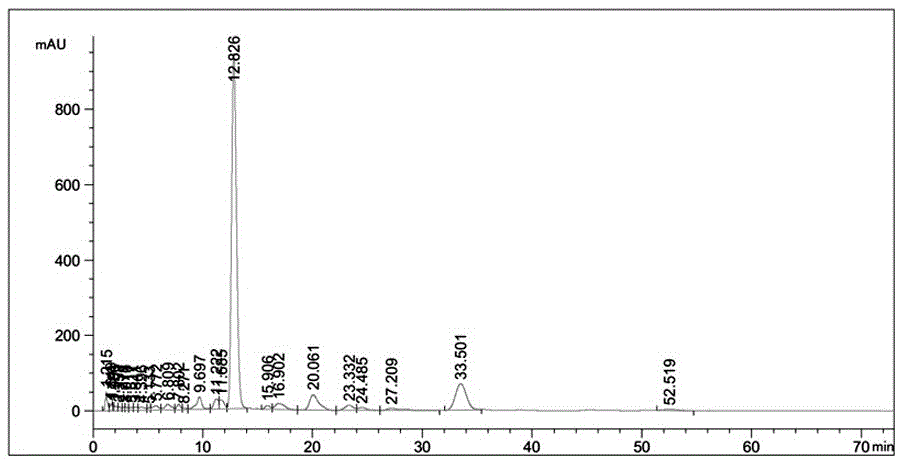
[0035] Get 110g Abamectin ointment, detect that its contained B1a composition is 5.06%; Add 200g methylene chloride in Abamectin ointment, be warming up to 50 ℃, stir, and Abamectin ointment is dissolved completely, A homogeneous solution was obtained; the resulting solution was cooled to 5° C., and 300 g of petroleum ether was added, and stirred. After crystallization began, the stirring was continued for 1 h to obtain a crystal slurry; the obtained crystal slurry was filtered under normal temperature to obtain 93 g of powder. Use HPLC to detect and analyze the powder and filtrate. The chromatographic detection conditions are: mobile phase (methanol: acetonitrile: water=38:38:24), wavelength (UV=245nm), chromatographic column (Zorbax C-18 4.6*150mm ), the pressure is 64bar, the test results are shown in Table 1 and attached figure 1 .
[0036] Table 1:
[0037]
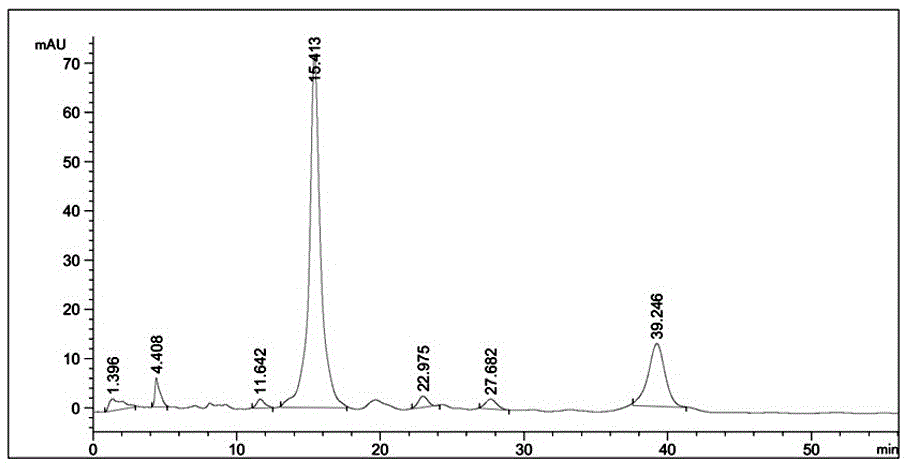
[0038] Abamectin standard sample spectrum such as figure 2 and shown in Table 2.
[0039] Table 2:
[00...
Embodiment 2
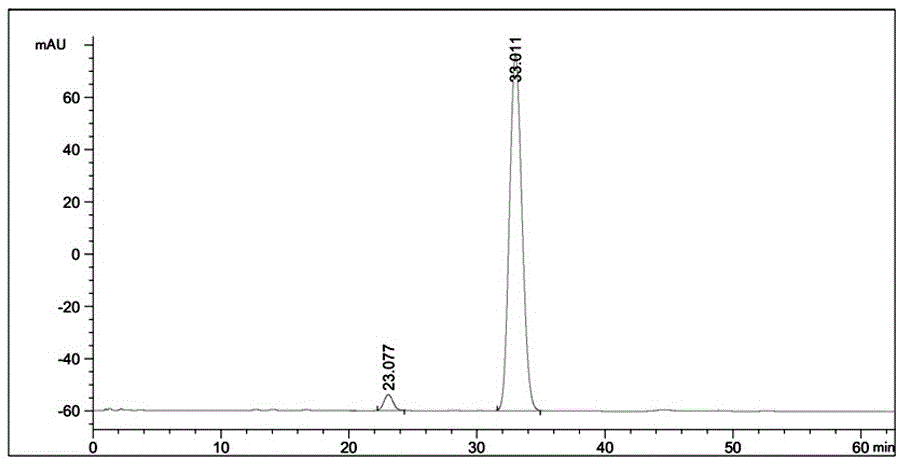
[0046] Get 1kg Abamectin Ointment, and detect that the contained B1a active ingredient is 5.06%; add 1kg bromoethane to Abamectin Ointment, heat up to 40°C, stir, and Abamectin Ointment is completely dissolved , to obtain a homogeneous solution; the resulting solution was cooled to 5 ° C, and 2 kg of n-pentane and n-hexane (miscible in any ratio) were added, stirred, and after the crystallization began, continued stirring for 1 h to obtain a crystal slurry; the obtained crystal slurry was filtered by suction , to obtain 0.91kg powder. The powder is detected and analyzed by HPLC, and the detection results are shown in Table 4 and image 3 .
[0047] Table 4:
[0048]
[0049] Abamectin B1a standard sample is used to calculate the absolute content of B1a in the powder by external standard method in Table 5.
[0050] table 5:
[0051]
[0052] The yield of abamectin B1a in the crystalline powder is 96.6%.
Embodiment 3
[0054] Get 113.2g of Abamectin ointment, and detect that the contained B1a component is 5.06%; add 80g of dichloropropane to Abamectin ointment, heat up to 50°C, stir, and make Abamectin ointment dissolve completely , to obtain a homogeneous solution; the resulting solution was cooled to 5 ° C, and 80 g of n-hexane was added, stirred, and after the crystallization began, the stirring was continued for 1 h to obtain a crystal slurry; the obtained crystal slurry was filtered under normal temperature to obtain 90 g of powder. Use HPLC to detect and analyze the powder, and the chromatographic detection conditions are: mobile phase (acetonitrile: water=80: 20), wavelength (UV=245nm), chromatographic column (Zorbax C-18 4.6*150mm), pressure is 53bar, FLOW=1.0ml / min test results are shown in Table 6 and Figure 5 :
[0055] Table 6
[0056]
[0057] Abamectin B1a standard sample is used to calculate the absolute content of B1a in the powder by external standard method in Table 7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com