Protein grafted copolymer and preparation method thereof
A technology of graft copolymer and protein, which is applied in the field of protein graft copolymer, can solve the problems that cannot meet the performance requirements of drug carrier materials, protein cannot exist stably for a long time, immunogenicity, long circulation time in the body, etc., and achieve simple Rapid preparation, long preparation cycle, complex post-processing effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1: the preparation of the keratin graft polyethylene glycol (Keratin-g-PEG) of graft rate 50%
[0039] Disperse 200mg of keratin in 4mL of water, then slowly add the initiator potassium persulfate (K 2 S 2 o 8 , 22mg) and 640mg MPEGMA, to be K 2 S 2 o 8 After fully dissolving with MPEGMA, the reaction was carried out at 70°C for 24h under continuous stirring. After the reaction, a dialysis bag with a molecular weight cut-off of 7 kDa was used to dialyze in water for 24 hours, and then freeze-dried to obtain a solid keratin-g-PEG graft copolymer with a graft rate of 50%.
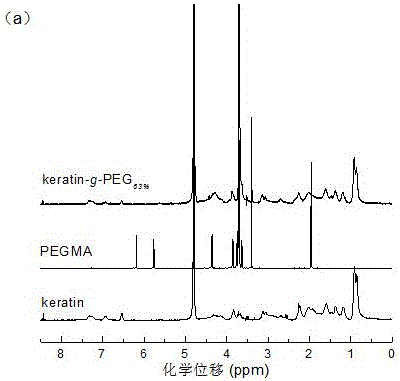
[0040] As can be seen from Figure 1(a), the proton peak at δ=3.75ppm in the Keratin-g-PEG NMR spectrum with a grafting rate of 50% belongs to the MPEGMA –CH 2 CH 2 The proton peak of O– indicates that the PEG chain has been successfully grafted onto the keratin backbone. By adjusting the feed ratio of MPEGMA and keratin, graft copolymers with different grafting ratios c...
Embodiment 2
[0045] Embodiment 2: the preparation of grafting rate 43% keratin graft poly-N-(2-hydroxypropyl) methacrylamide (keratin-g-PHPMA)
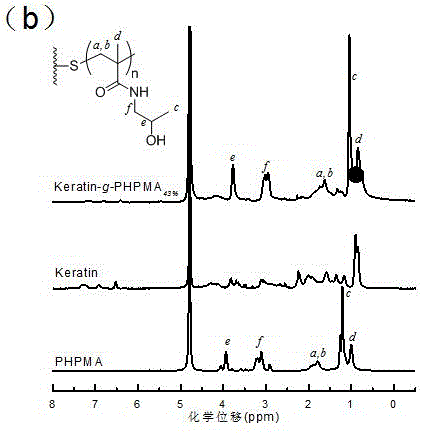
[0046] Disperse 200 mg of keratin in 4 mL of water, then add 4 mg of initiator 2,2'-azobisisobutyl ether hydrochloride (V-50) and 286 mg of N-(2-hydroxypropyl)methyl Acrylamide (HPMA). The reaction was then carried out at 50°C for 24 hours with constant stirring. After the reaction, the reactant was poured into a dialysis bag with a molecular weight cut-off of 7 kDa, dialyzed in water for 24 hours to remove the initiator and unreacted monomer, and the water was changed every 12 hours. After the dialysis was completed, a solid keratin-g-PHPMA graft copolymer with a graft ratio of 43% was obtained by freeze-drying. keratin-g-PHPMA in Figure 1(b) 43% The proton peaks at 3.9 and 3.2 ppm on the H NMR spectrum are derived from proton H at e and f marked on PHPMA, indicating that PHPMA has been successfully grafted onto the keratin main chain. By...
Embodiment 3
[0051] Embodiment 3: the preparation of grafting rate 62% keratin graft poly-N-isopropylacrylamide (keratin-g-PNIPAM)
[0052] 100 mg of keratin was dispersed in 4 ml of Milli-Q water, followed by the addition of 4 mg of Initiator V-50 and 670 mg of N-isopropylacrylamide (NIPAM). The reaction was carried out at 50°C under constant stirring for 24 hours, then the reactant was poured into a dialysis bag with a molecular weight cut-off of 7kDa, and dialyzed in water for 24 hours to remove the initiator and unreacted monomer, and the water was changed every 12 hours. After the dialysis was completed, the keratin-g-PNIPAM graft copolymer with a graft rate of 53% was obtained by freeze-drying.
[0053] The proton peak at 1.2ppm on the keratin-g-PNIPAM NMR spectrum in Figure 1(c) comes from -CH on PNIPAM 3 The proton H on , the proton peak in the range of 1.5-2.5ppm is derived from the PNIPAM repeating unit -CH 2 The proton on CH-, the proton peak of 3.8ppm is attributed to...
PUM
| Property | Measurement | Unit |
|---|---|---|
| critical micelle concentration (mass) | aaaaa | aaaaa |
| critical micelle concentration (mass) | aaaaa | aaaaa |
| degree of grafting | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com