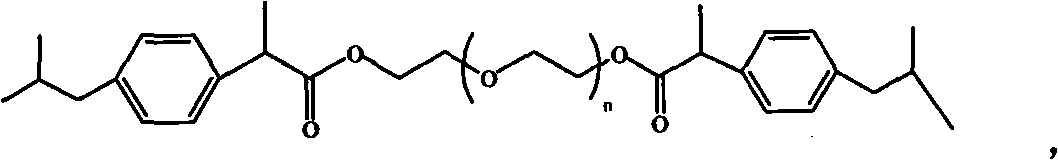
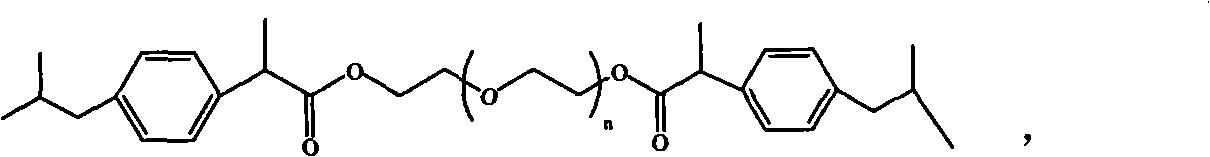
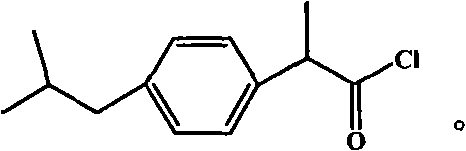
Ibuprofen end group-containing polyethylene glycol medicine macromonomer and synthesis method thereof
A polyethylene glycol and macromonomer technology, which is applied in the directions of drug combinations, pharmaceutical formulations, and medical preparations containing active ingredients, can solve the problems of high price of polyethylene glycol, difficult preparation, and gastrointestinal irritation, etc. Achieve the effect of not easily adhering to the blood vessel wall, mild operating conditions, and long half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] In the first step, the consumption of polyethylene glycol, solvent, ibuprofen acid chloride and catalyzer is respectively 9.6g, 13.2g, 2.25g and 0.8g, reacts 24 hours at room temperature, polyethylene glycol: solvent: ibuprofen Acyl chloride: the weight ratio of catalyzer is 1: 1.375: 0.234: 0.0833, and n is 89, and solvent is dichloromethane, and catalyzer is triethylamine; Ethylene glycol drug macromer is 9.56g, and the yield is 91.0%.
Embodiment 2
[0031] In the first step, the consumption of polyethylene glycol, solvent, ibuprofen acid chloride and catalyzer is respectively 9.6g, 8.79g, 2.25g and 2.54g, reacts 12 hours under 80 ℃, polyethylene glycol: solvent: ibuprofen Fenyl chloride: the weight ratio of catalyzer is 1: 0.916: 0.234: 0.265, and n is 136, and solvent is dichloromethane, and catalyzer is triethylamine; 7.64g of polyethylene glycol drug macromonomer, the yield is 72.7%.
Embodiment 3
[0033] In the first step, the consumption of polyethylene glycol, solvent, ibuprofen acid chloride and catalyzer is respectively 4.8g, 13.2g, 2.25g and 0.8g, reacts at room temperature for 36 hours, obtains the polyethylene glycol containing ibuprofen end group. The crude product of glycol drug macromer, polyethylene glycol: solvent: ibuprofen acid chloride: the weight ratio of catalyzer is 1: 2.75: 0.468: 0.167, and n is 45, and solvent is dichloromethane, and catalyzer is triethylamine; In the second step, 4.56 g of a white powdery product, i.e. polyethylene glycol drug macromonomer containing ibuprofen end groups, was obtained with a yield of 80%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com