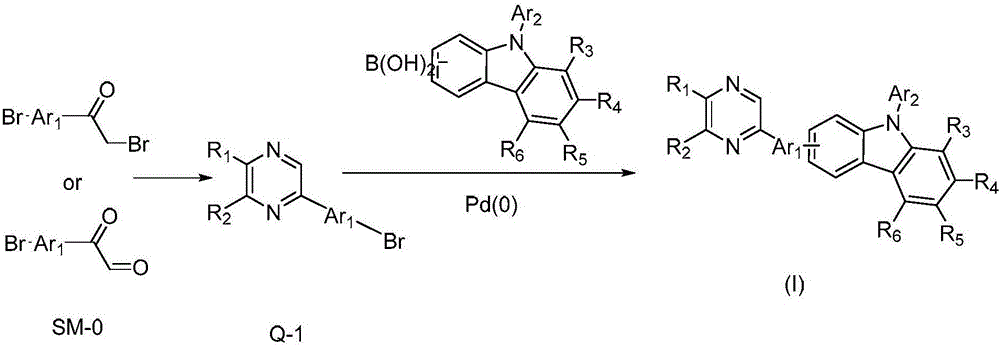
Pyrazine derivative and application thereof in organic electroluminescence device
A compound, C6-C60 technology, applied in the field of organic electroluminescence display
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] Embodiment 1, the preparation of compound SLC-1
[0074]
[0075] The first step: the preparation of intermediate Q-1
[0076]
[0077] 10g (46.9mmol) of 4-bromophenylacetone aldehyde was dissolved in 100ml of methanol-water, 5g (46.9mmol) of diaminomaleic cyanide was added, stirred at room temperature for 1 hour, 0.5g of potassium tert-butoxide was added, stirred Reacted for 24 hours, filtered, and the filtrate was concentrated to dryness under reduced pressure, separated and purified by silica gel column, and concentrated to dryness under reduced pressure to obtain 11.6 g of yellow solid with a yield of 87%.
[0078] The second step: the preparation of compound formula SLC-1
[0079]
[0080] Mix 10g (35mmol) of intermediate Q-1, 11g (38.6mmol) of 9-phenyl-3-carbazoleboronic acid, 7.4g (69.8mmol) of sodium carbonate, and then add 202mg (0.17mmol) of Pd(PPh 3 ) 4 Catalyst and 100mL of toluene, then add 20mL of ethanol and 20mL of water, under the protection...
Embodiment 2
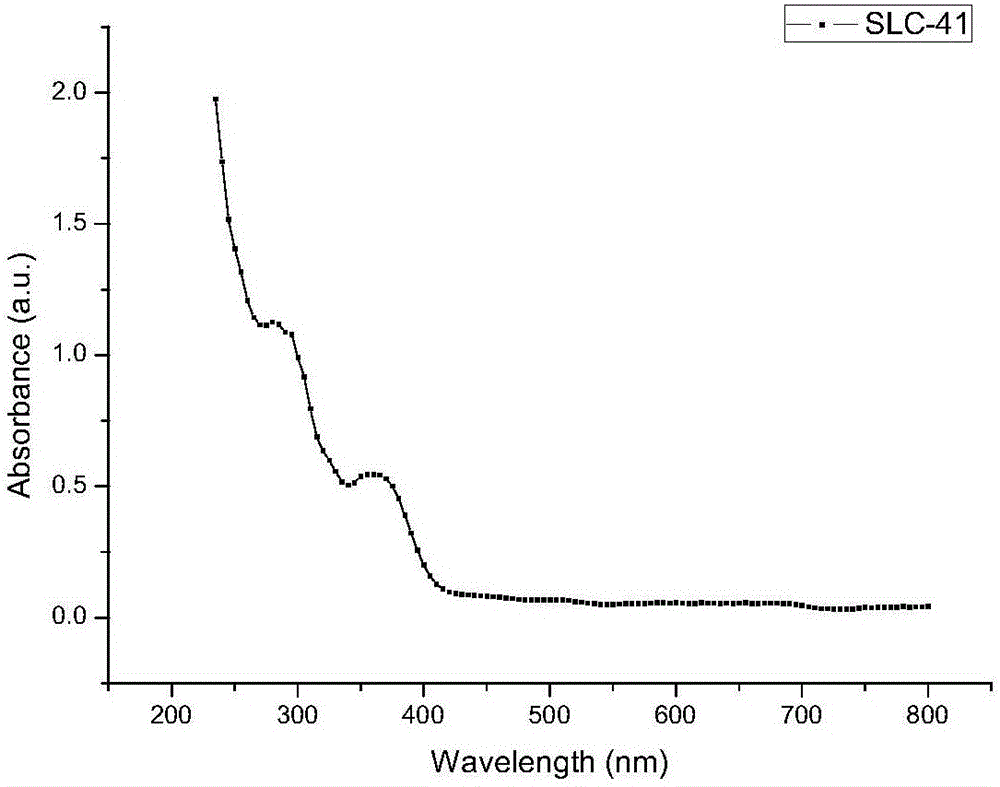
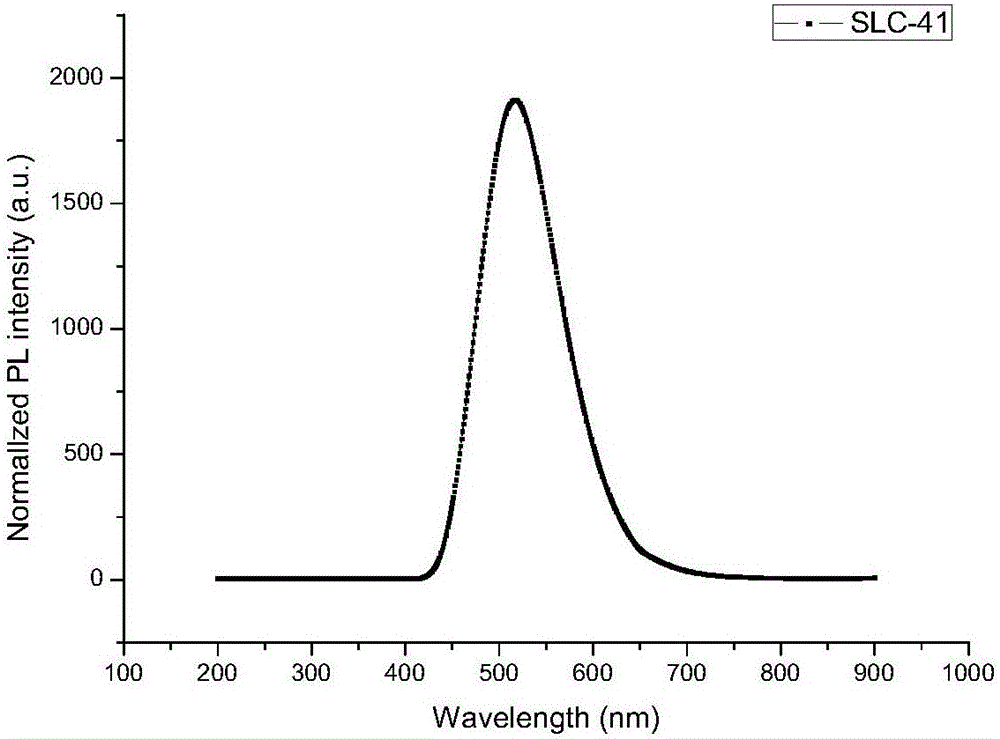
[0081] Embodiment 2, the preparation of compound SLC-41
[0082]
[0083] The first step: the preparation of intermediate Q-1
[0084]
[0085] 10g (36mmol) of p-bromophenyl bromoethanone was dissolved in 80ml of dry tetrahydrofuran, and under nitrogen protection, 3.9g (36mmol) of o-phenylenediamine was added, and then 0.5ml of pyridine was added, stirred at room temperature for 24 hours, filtered, The filtrate was concentrated to dryness under reduced pressure, separated and purified by a silica gel column to obtain 9.4 g of a yellow solid with a yield of 92%.
[0086] The second step: the preparation of compound formula SLC-41
[0087]
[0088] Mix 5g (17.5mmol) of intermediate Q-1, 5.5g (19.3mmol) of 9-phenyl-3-carbazole boronic acid, and 3.7g (34.9mmol) of sodium carbonate, and then add 100mg (0.086mmol) of Pd (PPh 3 ) 4 Catalyst and 60mL of toluene, then add 20mL of ethanol and 20mL of water, under the protection of nitrogen, raise the temperature and reflux ...
Embodiment 3
[0089] Embodiment 3, the preparation of compound formula SLC-83
[0090]
[0091] The synthetic operation refers to the second step of Example 2, 5g (17.5mmol) of intermediate Q-1, 7.8g (19.3mmol) of intermediate FPC-1 replaces the 9-phenyl-3- Carbazole boronic acid was separated and purified on a silica gel column to obtain 8.5 g of a yellow solid with a yield of 86%. HRMS: C 41 h 29 N 3 , standard molecular weight 563.24, test result 564.26.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Luminous wavelength | aaaaa | aaaaa |
| Half width | aaaaa | aaaaa |
| Film thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com