Macrocyclic amide metal complex and preparation method and application thereof
A metal complex and macrocyclic amide technology, which is applied in the field of macrocyclic amide metal complexes and their preparation, can solve the problems of low utilization rate of repeated catalysis, low structural stability, high synthesis cost, etc., and achieve high repeatable catalysis efficiency, The effect of good raw material stability and short preparation route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
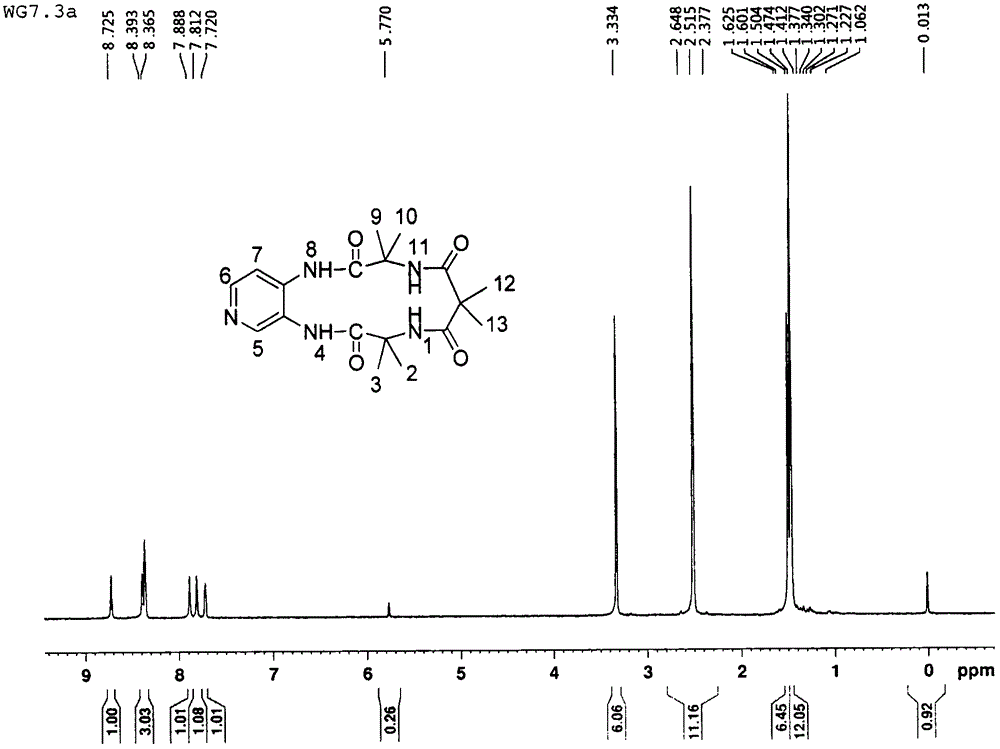
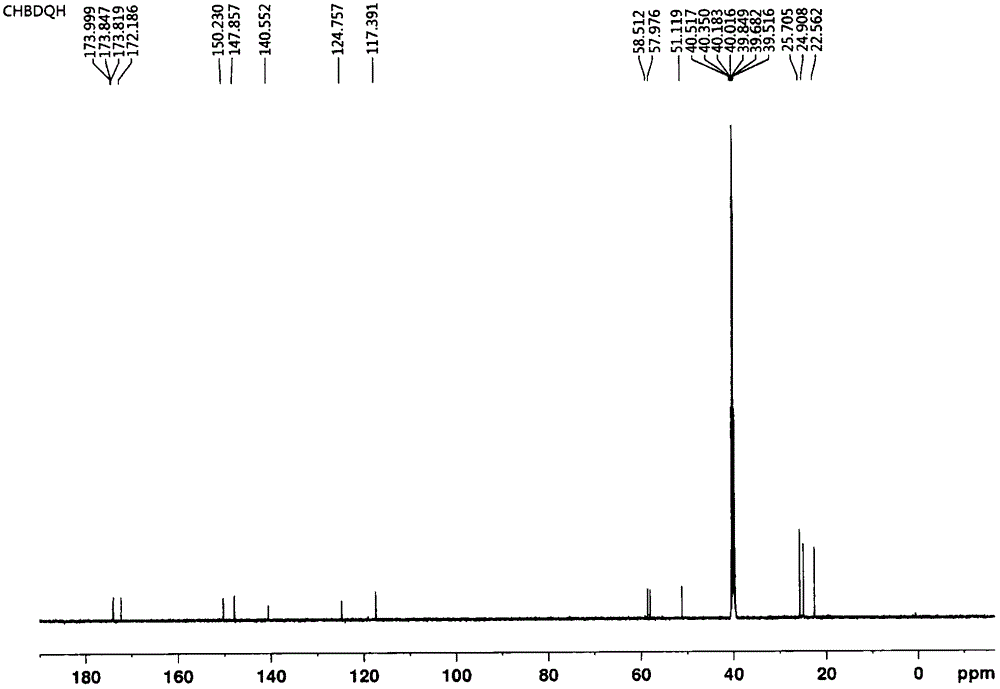
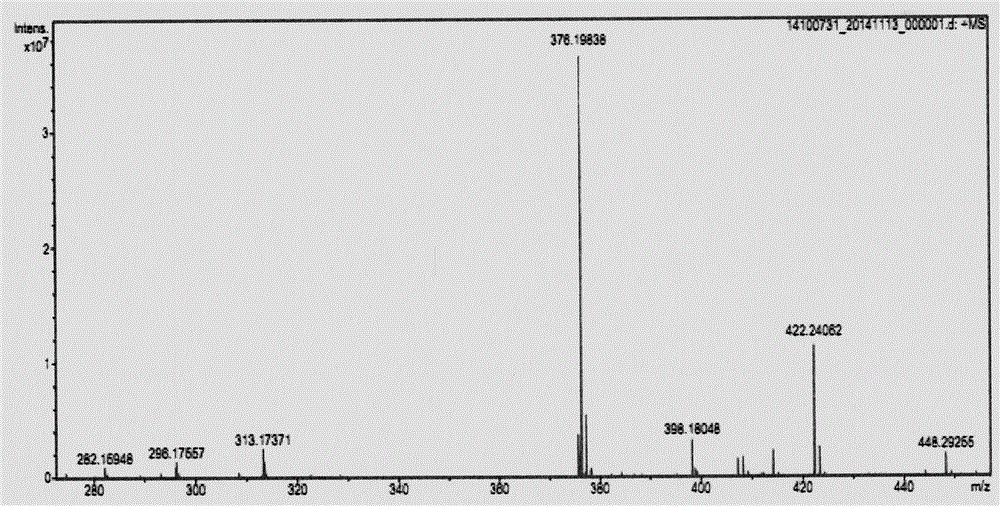
[0069] In a 250mL three-necked flask equipped with a constant pressure dropping funnel and argon protection, add a magnet and connect the argon protection, add 2-aminoisobutyric acid (10g, 97mmol), anhydrous pyridine (150mL), and stir at room temperature. Next, slowly add freshly prepared dimethylmalonyl chloride (8.2mL, 63mmol) into the constant pressure dropping funnel. After 1h, the dripping is completed. The temperature is raised to 65℃. After reaction for 12h, the pyridine is removed on the rotary evaporator to obtain an oily substance. 3M hydrochloric acid (30 mL) was adjusted to pH 2 to 3, and a large amount of solids precipitated. After standing at low temperature for 1 h, the filter cake was washed three times with a small amount of acetonitrile to obtain a white solid powder that was dried and weighed 11.6 g. The yield was 75%.
[0070] In a 100 mL three-necked flask equipped with a constant pressure dropping funnel and argon protection, add magnets, dimethylmalonamide d...
Embodiment 2
[0081] In a stainless steel pressure-resistant reactor (50mL), add magnets, 2-aminoisobutyric acid (4g, 38.8mmol), anhydrous pyridine (30mL), and diethyl dimethylmalonate (6.4mL, 38.8 mmol), replace the system with argon for 3 times, close the reaction kettle, heat up to 200°C in an oil bath and maintain for 2h, remove the reaction solution after cooling, remove the pyridine on a rotary evaporator to obtain an oil, add 3M hydrochloric acid (15mL) to adjust At pH 2-3, a large amount of solid precipitated. After standing at low temperature for 1 hour, the filter cake was washed three times with a small amount of acetonitrile to obtain a white solid powder weighing 5.2 g after drying, and the yield was 83%.
[0082] In a 100mL three-necked flask equipped with a constant pressure dropping funnel and argon protection, add magnets, dimethylmalonamide diacid (3g, 9mmol) and 1,2-dichloroethane (30mL) obtained from above. , Start stirring, slowly add thionyl chloride (7.5 mL, 90 mmol) to ...
Embodiment 3
[0088] Under the protection of argon, the compound of formula II (200mg, 0.53mmol) and 60ml of absolutely dry dichloromethane were added to a 100ml flask, and magnetic stirring was added at room temperature to make it dispersed in the solution as uniformly as possible to obtain a white suspension. Use a syringe to suck 1M KHMDS tetrahydrofuran solution (2.28ml, 2.28mmol) and slowly add it to the system. The system immediately changed from a white suspension to an orange suspension. Anhydrous iron trichloride solid (112mg, 0.692mmol), the system immediately changed from orange-yellow to brown-black, stirred at room temperature overnight, and TLC monitored until the reaction was complete. Filter with suction and wash the filter cake with a small amount of dichloromethane. The filter cake is dissolved in isopropanol and filtered. The obtained filtrate is placed on a rotary evaporator and the solvent is evaporated. The solid is dissolved in water and filtered. The filtrate is evapor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com