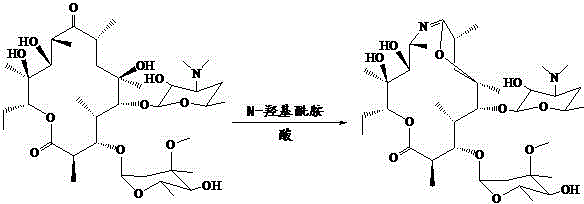
Preparation method of erythromycin 6,9-imino ether compound
A technology of imino ether and erythromycin, which is applied to the preparation of sugar derivatives, chemical instruments and methods, sugar derivatives, etc., to achieve the effects of industrial production, high yield, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Preparation of erythromycin 6, 9 imine ether compound: In an air atmosphere, add 10 mmol erythromycin thiocyanate, 10 mmol N-glycolamide and 50 ml acetonitrile to a 100 ml two-necked flask, and stir at room temperature for 10 minutes Finally, 3 mmol of concentrated sulfuric acid was added dropwise, and then a cooling tube was placed on the shelf and heated to 80°C with an oil bath under magnetic stirring, and reacted for 8 hours. Remove the oil bath, add 20 ml of water to the reaction solution, extract three times with 60 ml of ethyl acetate, combine the organic phases and wash with anhydrous MgSO 4 Dry for 30 minutes, filter; the filtrate is evaporated to dryness with a rotary evaporator, add water, adjust the pH value of the solution to 10-12 with NaOH solution, precipitate out, filter, and dry to obtain the erythromycin 6, 9 imino ether product with a yield of 86 %. The product has a melting point of 129-130°C. The nuclear magnetic analysis data are as follows: 1 ...
Embodiment 2
[0017] Preparation of erythromycin 6, 9 imine ether compound: Add 10 mmol erythromycin thiocyanate, 15 mmol N-hydroxypropionamide and 50 ml propionitrile to a 100 ml two-necked flask in an air atmosphere, and stir at room temperature After 10 minutes, 5 mmol of methanesulfonic acid was added dropwise, and then a cooling tube was placed on the shelf and heated to 100° C. with an oil bath under magnetic stirring, and reacted for 12 hours. Remove the oil bath, add 20 ml of water to the reaction solution, extract three times with 60 ml of ethyl acetate, combine the organic phases and wash with anhydrous MgSO 4 Dry for 30 minutes, filter; the filtrate is evaporated to dryness with a rotary evaporator, add water, adjust the pH value of the solution to 10-12 with NaOH solution, precipitate out, filter, and dry to obtain the erythromycin 6, 9 imino ether product with a yield of 83 %.
Embodiment 3
[0019] Preparation of erythromycin 6, 9 imine ether compound: in air atmosphere, add 10mmol erythromycin thiocyanate, 16 mmol N-hydroxybenzamide and 50 ml dioxane to a 100 ml two-necked flask, After stirring at room temperature for 10 minutes, 4 mmol of benzenesulfonic acid was added dropwise, and then a cooling tube was placed on the rack and heated to 110° C. with an oil bath under magnetic stirring, and reacted for 24 hours. Remove the oil bath, add 20 ml of water to the reaction solution, extract three times with 60 ml of ethyl acetate, combine the organic phases and wash with anhydrous MgSO 4 Dry for 30 minutes, filter; the filtrate is evaporated to dryness with a rotary evaporator, add water, adjust the pH value of the solution to 10-12 with NaOH solution, precipitate out, filter, and dry to obtain the erythromycin 6, 9 imino ether product with a yield of 80 %.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com