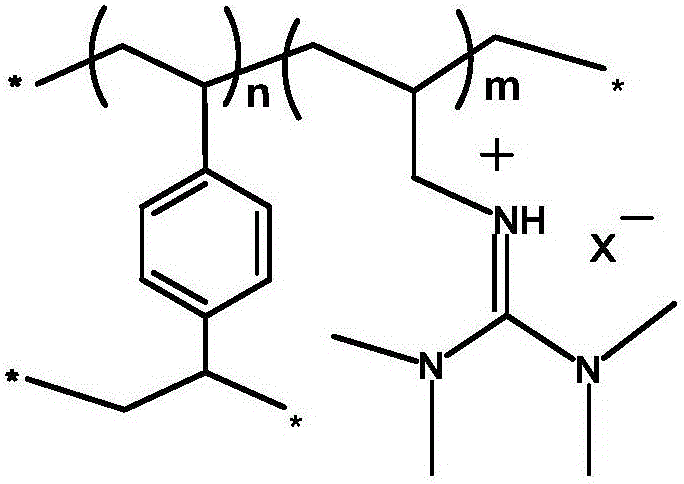
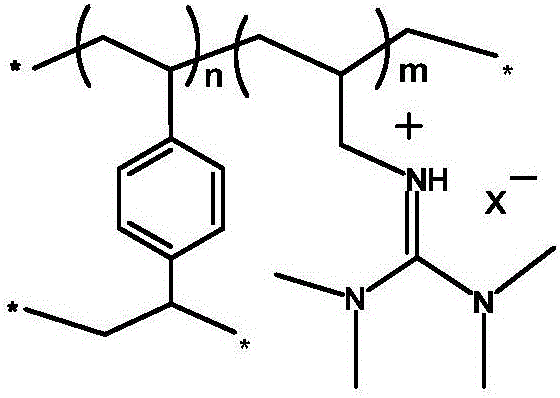
Ion-type macromolecular adsorbent for adsorbing Pd<2+> metal ions and preparation method of ion-type macromolecular adsorbent
A technology of metal ions and polymers, applied in the field of environmental protection and material science, to achieve the effect of simple preparation process, strong adsorption capacity, and high saturated adsorption capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] A. Mix tetramethylguanidine and 3-bromopropene ethanol solution according to the stoichiometric ratio, stir and react at 80°C for 24h, then remove the solvent under reduced pressure, wash and dry to obtain the guanidine ionic liquid precursor;
[0020] B. the above-mentioned synthesized guanidine ionic liquid precursor and divinylbenzene are added to 100mL ethanol according to the molar ratio of 1:1, then add a certain amount of initiator azobisisobutyronitrile, whose mass fraction is guanidine 1% of the total mass of the ionic liquid precursor and divinylbenzene, stirred at 80°C for 24h, filtered, washed and dried to obtain a bromide ion-type polymer;
[0021] C. Add the above-mentioned synthesized bromide ion-type macromolecule and anion precursor sodium hexafluorophosphate into a certain amount of deionized water according to the stoichiometric ratio, stir at room temperature for 48 hours, filter, wash and dry to obtain hexafluorophosphate ion-type high molecular ads...
Embodiment 2
[0024] The operation method is the same as in Example 1, except that the anion precursor sodium hexafluorophosphate in step C is replaced with sodium tetrafluoroborate to obtain tetrafluoroborate ionic polymer adsorbent.
Embodiment 3
[0026] The operation method is the same as in Example 1, except that the anion precursor sodium hexafluorophosphate in step C is replaced with acetic acid to obtain the acetate ion-type polymer adsorbent.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com