Method for producing D-1,2,4-butantriol through bio-converting cellulosic hydrolyzate
A cellulose hydrolyzate and biotransformation technology, applied in the biological field, can solve the problem of low yield, and achieve the effects of improving purity, saving costs, and reducing bypass pathways.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1 Construction of genetically engineered bacteria
[0026] Construction of clones expressing 2-ketoacid decarboxylase (mdlC), D-xylose dehydrogenase (xylB), xylose dehydratase (yjhG) and alcohol dehydrogenase (adhP), knockout host bacteria for xylose utilization and D -BT synthesizes the xylose isomerase (xylA) gene in the intermediate metabolite decomposition pathway, and obtains genetically engineered bacteria, among which, 2-ketoacid decarboxylase (mdlC), GenBank: AY143338.1; D-xylose dehydrogenase (xylB), Gene ID: 7329904; Xylose Dehydratase (yjhG), Gene ID: 946829; Alcohol Dehydrogenase (adhP), Gene ID: 00 946036; Xylose Isomerase (xylA), Gene ID: 948141 .
Embodiment 2
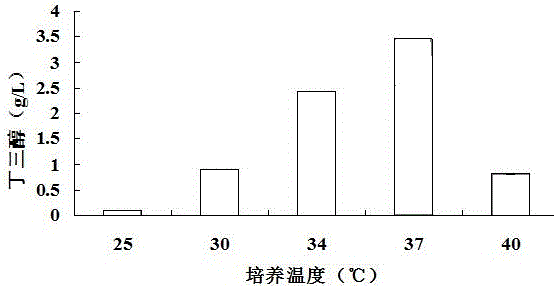
[0027] Example 2 Effects of Different Fermentation Temperatures on the Production of Butanetriol Using Corncob Hydrolyzate as Substrate
[0028] The suspended hydrolyzate mixed with 2% (v / v) sulfuric acid and corn cob hydrolyzate at a ratio of 1:5 (w / v) was sterilized in an autoclave at 121°C for 20 minutes. Add alkaline reagent Ca(OH) 2 To neutralize sulfuric acid, the added Ca(OH) 2 The concentration is equal to the number of moles of the same sulfuric acid concentration, and NaOH is added to adjust the pH to 7.2. Finally, use filter paper to filter out the solid matter in the corn cob hydrolyzate pretreatment method, then add 2% (w / v) activated carbon to the corn cob hydrolyzate, heat at 50°C for 30 minutes, and then use filter paper to filter out the activated carbon to obtain clarification corncob hydrolyzate. The concentration of xylose in the obtained corncob hydrolyzate is 44g / L, add 10g / L Nacl, 5g / L yeast powder and 10g / L peptone in every liter of corncob hydrolyza...
Embodiment 3
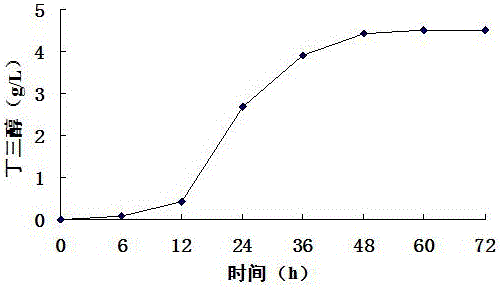
[0029] Example 3 The impact of buffer solution on the production of butanetriol with corncob hydrolyzate as substrate
[0030] The suspended hydrolyzate mixed with 2% (v / v) sulfuric acid and corn cob hydrolyzate at a ratio of 1:5 (w / v) was sterilized in an autoclave at 121°C for 20 minutes. Add alkaline reagent Ca(OH) 2 To neutralize sulfuric acid, the added Ca(OH) 2 The concentration is equal to the number of moles of the same sulfuric acid concentration, and NaOH is added to adjust the pH to 7.2. Finally, use filter paper to filter out the solid matter in the corncob hydrolyzate, then add 2% (w / v) activated carbon to the corncob hydrolyzate, heat at 50°C for 30 minutes, and then filter out the activated carbon with filter paper to obtain clarified corncob hydrolyzate liquid. The concentration of xylose measured in the clarified corncob hydrolyzate is 44g / L, and 10g / L Nacl, 5g / L yeast powder and 10g / L peptone are added in every liter of corncob hydrolyzate, sterilized, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com