Quality control method of Periplaneta americana freeze-dried powder
A quality control method, the technology of Periplaneta americana, applied in the direction of measuring devices, preparation of test samples, color/spectral characteristics measurement, etc., can solve the problems of serious side effects, over-treatment, deterioration of the patient's body condition, etc., and achieve quality assurance and curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1 Amino Acid Identification
[0047] Weigh 0.5g of Periplaneta americana freeze-dried powder, add 10ml of 70% ethanol, sonicate for 10min, filter, collect the filtrate, evaporate to dryness in a water bath, add 5ml of 70% ethanol to dissolve the residue, and use it as the test solution.
[0048] Take alanine, phenylalanine, and glycine reference substances respectively, accurately weigh them, put them in a brown measuring bottle, add 70% ethanol to make a 1mg / ml reference substance solution.
[0049] According to the thin-layer chromatography test, draw 1 μl of each of the above four solutions, spot them on the same silica gel G thin-layer plate, use n-butanol-formic acid-water (75:15:10) as the developing solvent, and develop after saturation for 30 minutes. Take it out, dry it, spray it with 0.2% ninhydrin ethanol solution, heat it at 105°C until the spots are clear, and inspect it under sunlight.
[0050] The result is as figure 1 As shown, among the figure...
Embodiment 2
[0051] Embodiment 2 Determination of polypeptide content
[0052] The main active ingredients in Periplaneta americana freeze-dried powder are polypeptides. According to the relevant regulations under the 2010 edition of "Chinese Pharmacopoeia", Appendix VA UV-visible spectrophotometry, refer to the literature and use bovine serum albumin as a reference substance to determine the total polypeptides content.
[0053] 2.1 Preparation of reference solution
[0054] Accurately weigh 51.80mg of bovine serum albumin reference substance (BSA), put it in a 100ml volumetric flask, add distilled water to dissolve to the mark, shake well, and prepare a reference substance solution containing 0.5180mg bovine serum albumin per 1ml.
[0055] 2.2 Selection of detection wavelength
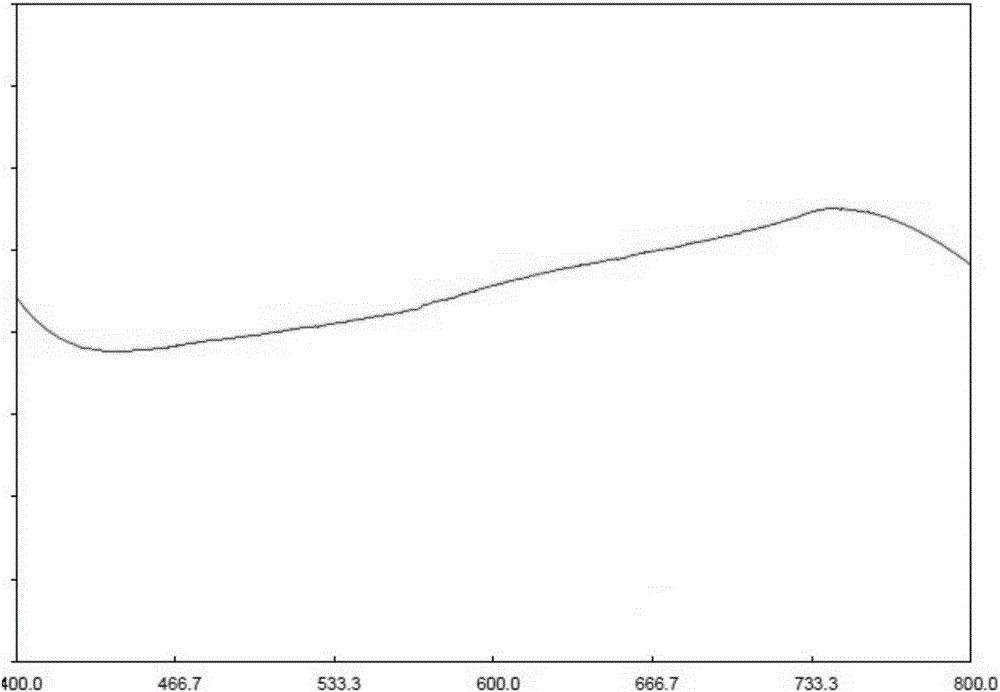
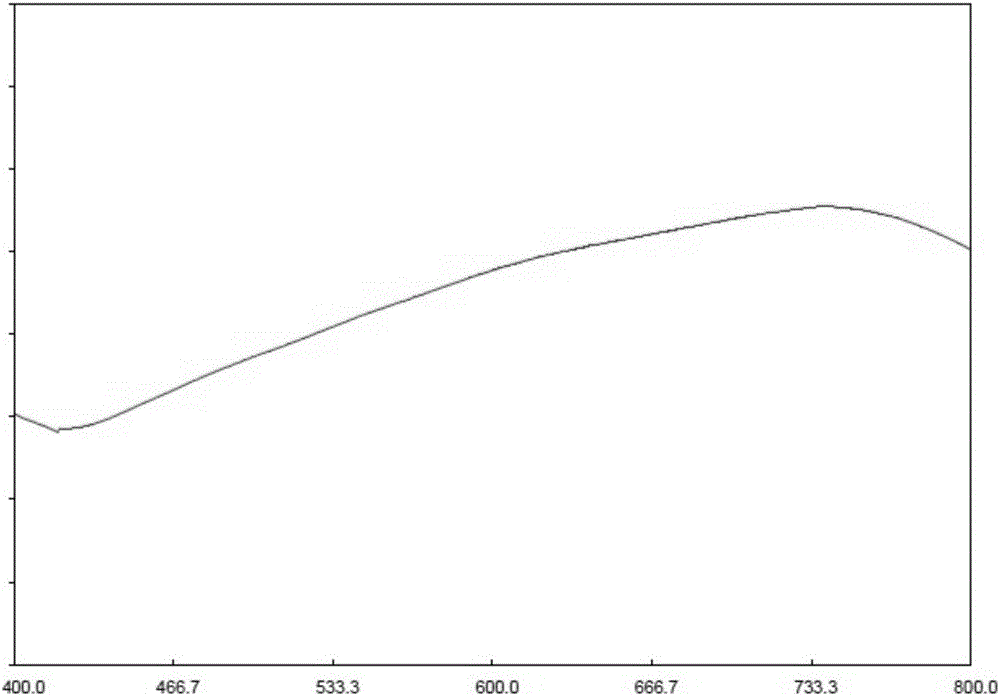
[0056] Precisely measure the reference substance solution, put 1ml of the sample solution into a 10ml graduated test tube with stopper, add 5.0ml of alkaline copper reagent, mix well, quickly add 0.5ml of phenol...
Embodiment 3
[0111] Embodiment 3 Amino Acid Content Determination
[0112] In Periplaneta americana freeze-dried powder, amino acid is one of the active ingredients, according to the relevant regulations under the 2010 edition of "Chinese Pharmacopoeia" one appendix VA ultraviolet-visible spectrophotometry, with alanine as a reference substance to measure the total amino acid content.
[0113] 3.1 Preparation of reference solution
[0114] Accurately weigh 16.08mg of alanine reference substance, put it in a 100ml volumetric flask, add 10% isopropanol to dissolve, and dilute to the mark as mother liquor. Accurately measure 4ml, put it in a 50ml volumetric flask, and bring it to the mark with water to prepare a reference solution containing 12.86μg alanine per 1ml.
[0115] 3.2 Selection of detection wavelength
[0116] Accurately measure the reference substance solution and the sample solution in a 10ml graduated test tube with a stopper, add 0.1ml of freshly prepared 1% Vc solution, 3ml ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com