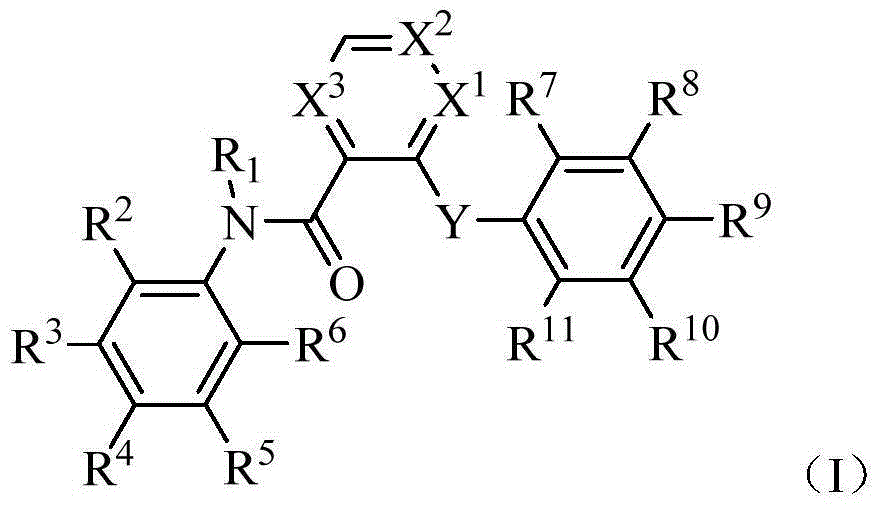
Heteroaryl amide derivative and use thereof as TGR5 agonist
A heteroaryl and heterocyclic group technology, applied in the direction of medical preparations containing active ingredients, drug combinations, digestive system, etc., can solve the problems of weakening GLP-1 secretion and increasing blood sugar levels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0115] The present invention also provides the preparation method of above-mentioned compound:
[0116] 1. Preparation of intermediate 1:
[0117] (1) When Y is an oxygen atom,
[0118]
[0119] Dissolve raw material 1, raw material 2, basic catalyst, and Cu(I) in an aprotic solvent, heat until the reaction is complete, cool to room temperature, filter, add water and an organic solvent (such as ethyl acetate, dichloromethane), Separate the liquid, wash the organic phase with a saturated aqueous sodium chloride solution, dry over anhydrous sodium sulfate, filter, concentrate, and purify by column chromatography to obtain an intermediate product, which is then subjected to a conventional deesterification reaction to obtain intermediate 1.
[0120] In this reaction, R' is selected from C 1-6 Alkyl, preferably C 1-4 alkyl, X is halogen; the Cu(I) source refers to copper(I) salts such as copper(I) bromide and copper(I) iodide or copper(I) which are typically more soluble in o...
experiment example 1
[0173] Experimental example 1 In vitro cell activity test of the compound of the present invention
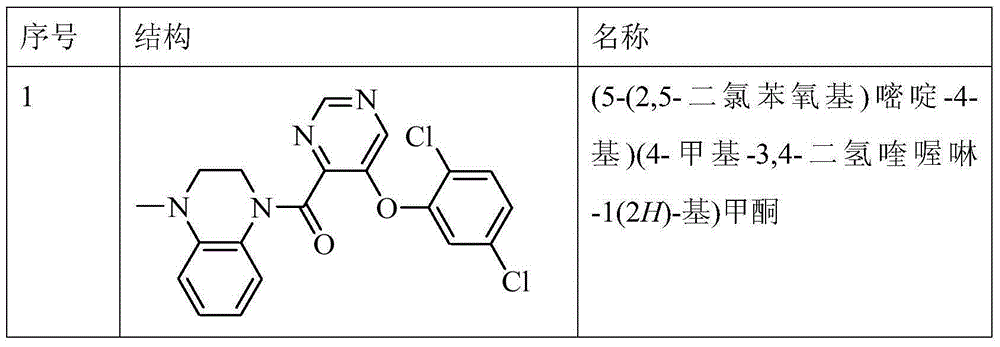
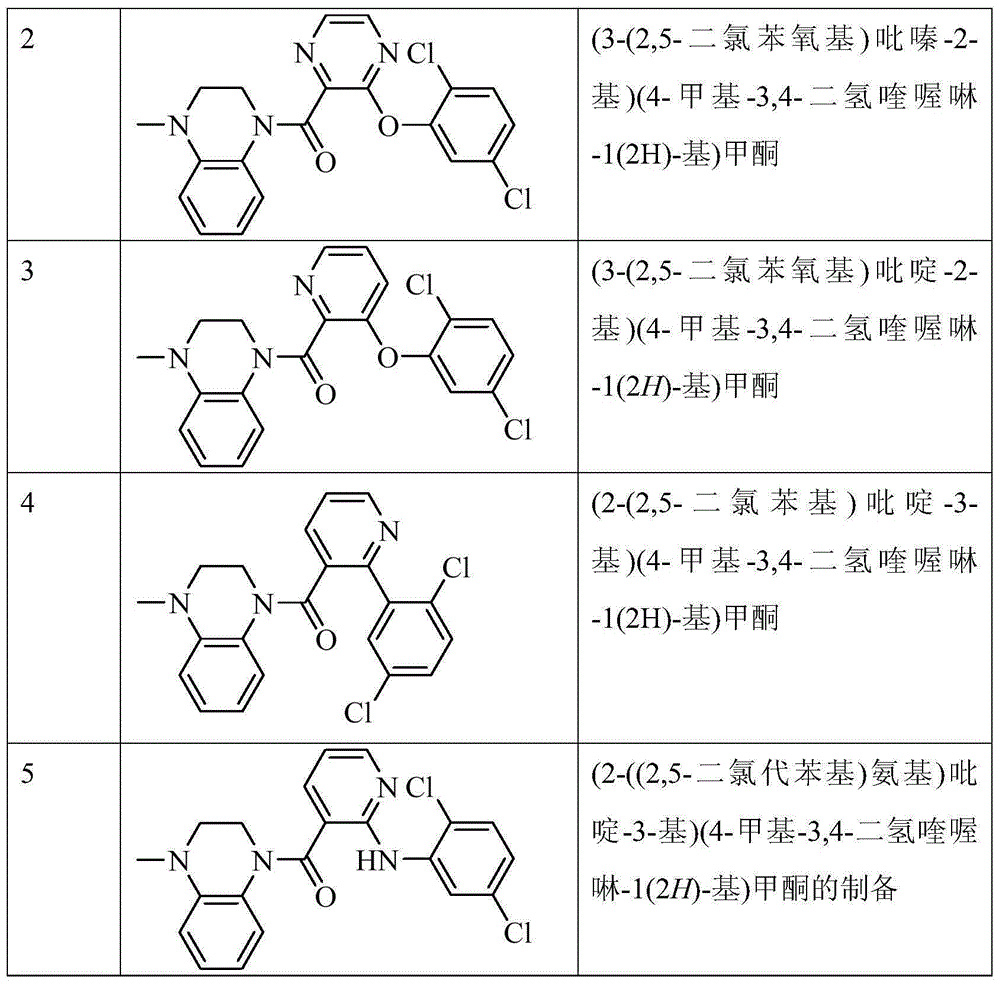
[0174] Test product: Compounds 1, 2, 3, 4 and 5 of the present invention, see the preparation examples of each compound for their chemical names and preparation methods.
[0175] The meanings represented by the abbreviations of the following experiments are as follows:
[0176] DMSO: dimethyl sulfoxide
[0177] GPBA: G protein-coupled bile acid receptor
[0178] CHO-K1: Chinese hamster ovary cell substrain
[0179] cAMP: cyclic adenosine monophosphate
[0180] homogeneous time-resolved fluorescence technique
[0181] Kit detection principle: Cyclic AMP assay kit is based on homogeneous time-resolved fluorescence technology ( ), intended to directly quantify the content of cyclic AMP in suspension or adherent cells. The detection method is based on the existence of competitive immunobinding between cAMP produced by cells and d2 (energy acceptor) dye-labeled cAMP and...
Embodiment 1
[0206] Example 1 Preparation of (5-(2,5-dichlorophenoxy)pyrimidin-4-yl)(4-methyl-3,4-dihydroquinoxalin-1(2H)-yl)methanone
[0207]
[0208] 1) Preparation of 5-bromopyrimidine-4-formyl chloride
[0209]
[0210] Dissolve 5-bromopyrimidine-4-carboxylic acid (0.6g, 2.96mmol) in thionyl chloride (10mL), add N,N-dimethylformamide (0.05mL), heat to 90°C and stir for 3 hours . The reaction solution was concentrated to dryness, added toluene (10 mL), and concentrated to dryness again, and the obtained crude product was directly used in the next reaction.
[0211] 2) Preparation of methyl 5-bromopyrimidine-4-carboxylate
[0212]
[0213] 5-Bromopyrimidine-4-carbonyl chloride (0.65 g, 2.94 mmol) obtained in the previous step was dissolved in anhydrous methanol (10 mL), and stirred at 25°C for 16 hours. The reaction solution was concentrated to dryness, and the obtained crude product was washed with petroleum ether to obtain the title compound (0.58g, yield 90.6%)
[0214] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com