A new type of water-soluble reactive ultraviolet absorber and preparation method thereof
A UV absorber and water-soluble technology, applied in chemical instruments and methods, other chemical processes, organic chemistry, etc., can solve problems such as poor reactivity and no active groups, and achieve simple methods, good water solubility, and wide application prospects Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
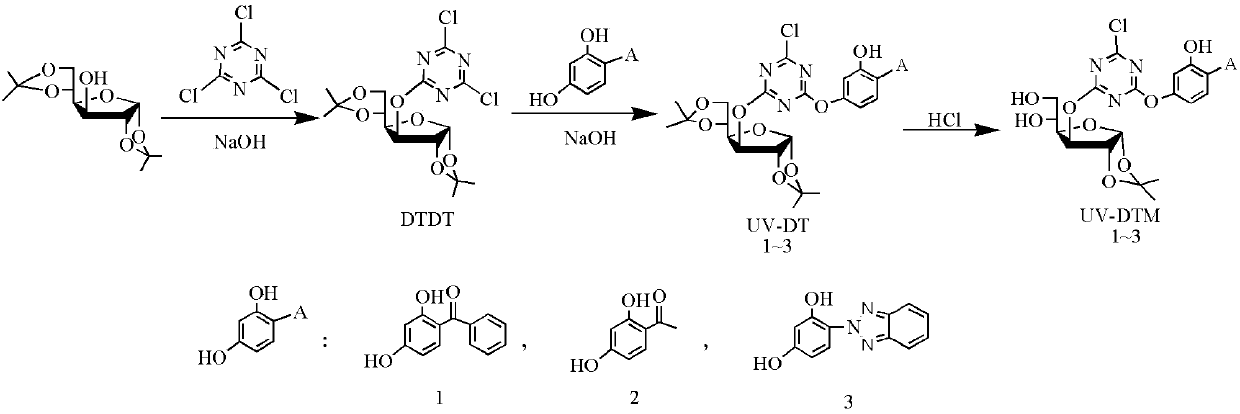
[0037] Example 1: 3-(4,6-Dichloro-1,3,5-triazine-2-oxy)-1,2,5,6-bisisopropylidene-α-D-glucose (DTDT) Synthesis
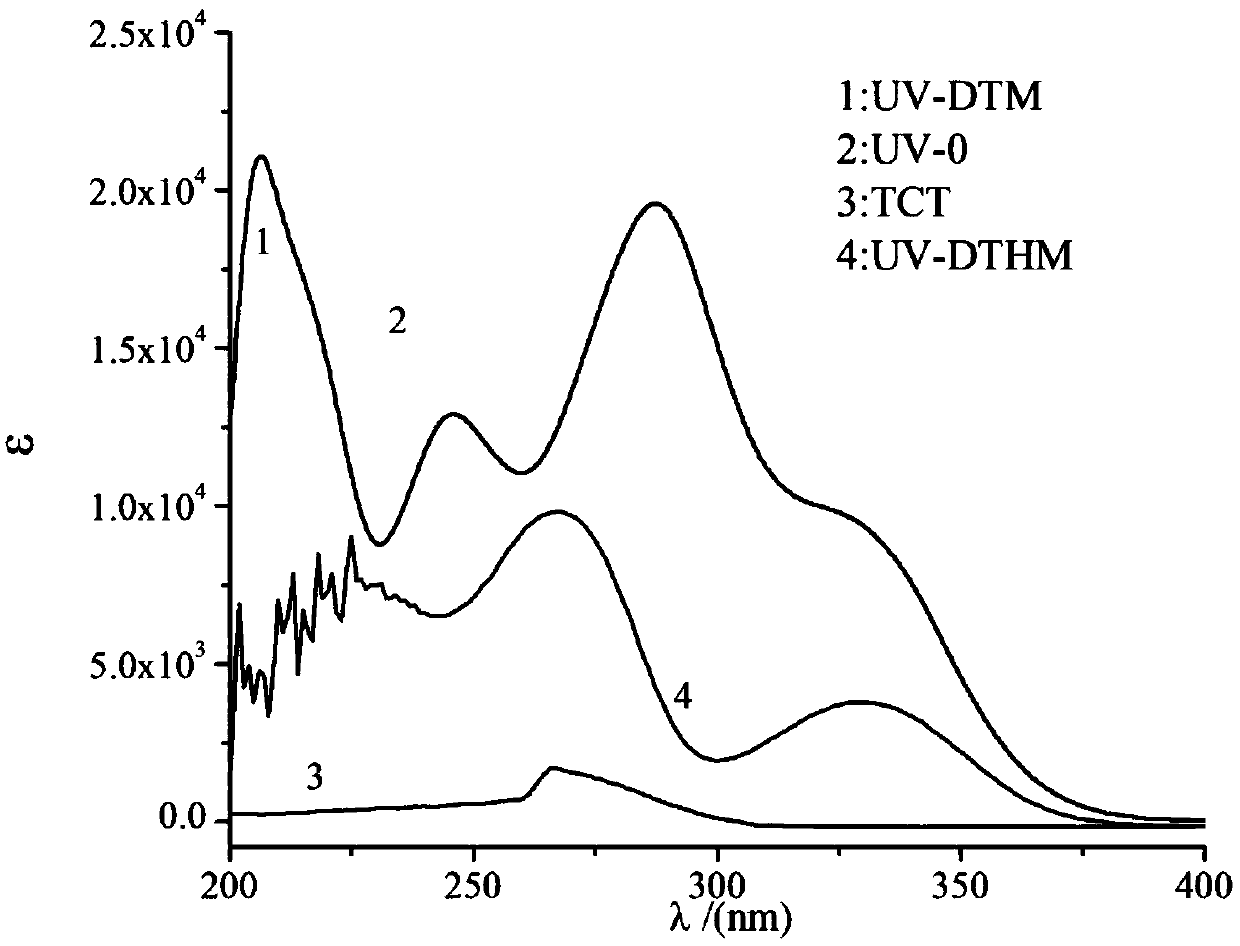
[0038]Dissolve 1.46g (8mmol) of cyanuric chloride (TCT) in 20mL of acetone, stir and swell at 0°C for 10min, and then slowly add an acetone-water mixed solution composed of diacetone glucose and NaOH dropwise to the solution. The mixed solution consisted of 2.60 g (10 mmol) of diacetone glucose dissolved in 20 mL of acetone, and a solution of water (10 mL) dissolved with 0.68 g (17 mmol) of NaOH was added dropwise with stirring at zero temperature. The reaction was followed by TLC. After the reaction was completed, the solvent was removed by rotary evaporation to obtain a light yellow oily product, which was purified by column chromatography (the eluent was ethyl acetate: petroleum ether=1:6) to obtain a white solid 3-(4,6-dichloro-1,3). ,5-triazine-2-oxy)-1,2,5,6-bisisopropylidene-α-D-glucose (DTDT). Yield: 73%.
[0039] The effects of alkali dosage, reaction t...
Embodiment 2
[0047] Example 2: 3-(2-(2-Hydroxyphenylbenzophenone-4-oxy)-4-chloro-1,3,5-triazine-6-oxy)-1,2,5, Synthesis of 6-diisopropylidene-α-D-glucose (UV-DT)
[0048] Dissolve 2.04g (5mmol) DTDT in 30mL acetone, mix 0.96g (4.5mmol) UV-0-dissolved acetone (20mL) and dissolve 0.20g (5mmol) NaOH aqueous solution (10mL), then slowly add dropwise into the DTDT solution. The whole system was reacted at 30°C for 2h. The solvent was removed by rotary evaporation, the remaining yellow solid was repeatedly washed with ethanol, and purified by column chromatography (the eluent was acetone: n-hexane = 1:1) to obtain a white powder 3-(2-(2-hydroxyphenylbenzophenone-4). -oxy)-4-chloro-1,3,5-triazine-6-oxy)-1,2,5,6-bisisopropylidene-α-D-glucose (UV-DT). Yield: 74%.
[0049] The substitution of the second chlorine of cyanuric chloride is usually carried out at about room temperature, and the reaction with the hydroxyl group requires the addition of alkali as a catalyst. Therefore, the reaction tem...
Embodiment 3
[0054] Example 3: 3-(2-(2-Hydroxyphenylbenzophenone-4-oxy)-4-chloro-1,3,5-triazine-6-oxy)-1,2-isopropyl Synthesis and Properties of Fork-α-D-glucose (UV-DTM)
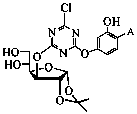
[0055] Dissolve 0.59 g (1 mmol) UV-DT in 10 mL of tetrahydrofuran, add 0.50 mL of hydrochloric acid (36%) solution, stir at room temperature for 5 hours, remove the solvent by rotary evaporation, and the crude product is subjected to column chromatography (the eluent is ethyl acetate). : petroleum ether=1:1) to obtain hydrolyzed product 3-(2-(2-hydroxyphenylbenzophenone-4-oxy)-4-chloro-1,3,5-triazine-6-oxyl )-1,2-isopropylidene-α-D-glucose (UV-DTM). Yield: 74%.
[0056] Propylene ketone protecting group can be deprotected under acidic conditions, but other ether bonds in the structure can also be disconnected when heated under acidic conditions, so adopt the method of TLC detection at room temperature, investigate different acids (acetic acid, hydrochloric acid, The effect of trifluoroacetic acid) catalysis on the hy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com