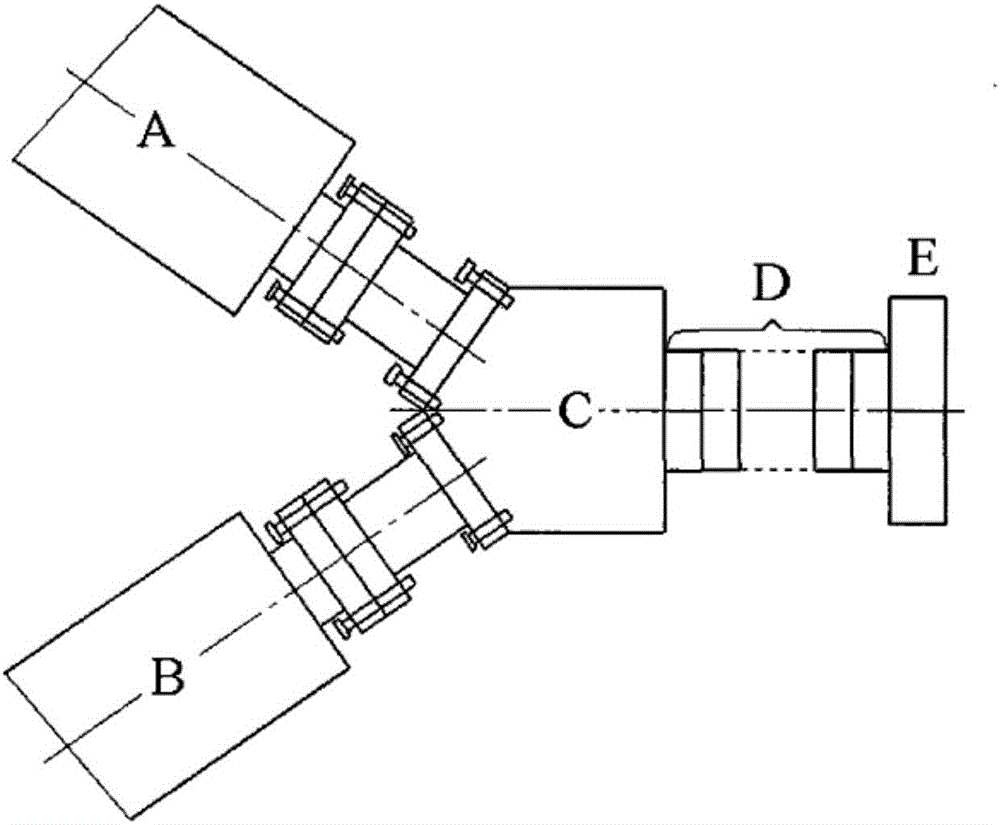
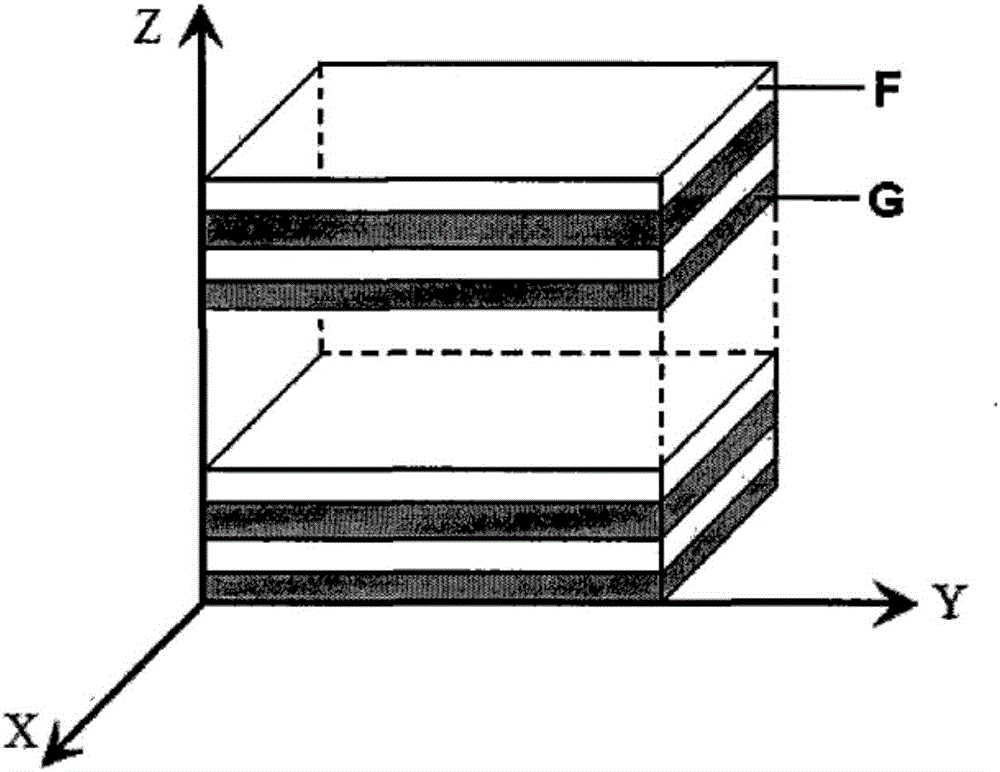
Preparation method of macromolecular laminar drug-loaded hydrogel with controllable drug distribution
A technology for polymer layer and drug preparation, which can be used in the fields of polymer compound inactive ingredients, drug delivery, and pharmaceutical formulations, etc., and can solve the problems of low drug concentration, complicated process, and long time consumption.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1 (drug-loaded layer A and barrier layer B)
[0046] In the first step, sodium alginate and water are 10%:90% by weight, calcium carbonate-gluconolactone system (a sodium alginate cross-linking agent, wherein the molar ratio of calcium carbonate and gluconolactone is 1:2) According to 15% of the weight of sodium alginate, the drug methylene blue is prepared according to the total weight of sodium alginate and water 10%, and the polyanion polymer blend solution A is obtained by degassing and stirring with a vacuum defoaming mixer.
[0047] Chitosan and water are mixed according to the weight percentage of 10%: 90%, and genipin (which is chitosan crosslinking agent) is prepared according to 10% of the mass of chitosan, and the vacuum defoaming mixer is degassed and stirred evenly to obtain poly Cationic polymer blend solution B.
[0048] In the second step, the above-mentioned uniformly mixed sodium alginate drug-loaded blend solution (polyanionic polymer blend s...
Embodiment 2
[0052] Example 2 (drug-loaded layer A and barrier layer B)
[0053] In the first step, the weight percentage of sodium alginate and water is 10%: 90%, calcium sulfate (as a sodium alginate cross-linking agent) is 20% of the weight of sodium alginate, and the drug ibuprofen is calculated by the total weight of sodium alginate and water. The weight is 10% for batching, and the vacuum defoaming mixer is degassed and stirred to obtain a polyanionic polymer drug-loaded blend solution A.
[0054] Chitosan and water are mixed according to the weight percentage of 10%: 90%, and genipin (which is chitosan crosslinking agent) is prepared according to 10% of the mass of chitosan, and the vacuum defoaming mixer is degassed and stirred evenly to obtain poly Cationic polymer blend solution B.
[0055] In the second step, the above-mentioned uniformly mixed sodium alginate blend solution (polyanionic polymer drug-loaded blend solution A) and chitosan blend solution (polycation polymer blend...
Embodiment 3
[0059] Example 3 (drug-loaded layer A and barrier layer B)
[0060] In the first step, the weight percentage of hyaluronic acid and water is 30%: 70%, glutaraldehyde (as a hyaluronic acid crosslinking agent) is 5% of the weight of hyaluronic acid, and the drug ibuprofen is mixed with hyaluronic acid and water. The total weight is 30% for batching, and the polyanionic polymer blend solution A is obtained by degassing and stirring with a vacuum defoaming mixer.
[0061] Mix gelatin and water at a weight percentage of 20%:80%, glutaraldehyde (gelatin cross-linking agent) according to 2% of the gelatin mass, and degass and stir with a vacuum defoaming mixer to obtain a polycationic polymer blend solution b.
[0062] In the second step, the above-mentioned homogeneously mixed hyaluronic acid blend solution (polyanionic polymer blend solution A) and gelatin blend solution (polycation polymer blend solution B) are respectively stirred by an extruder at 25°C extruded, and stacked to...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Width | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com