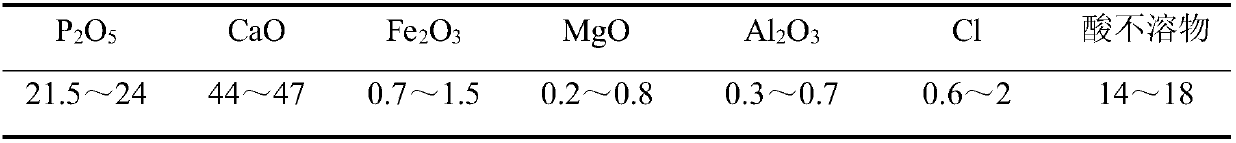A kind of extraction agent and extraction method of industrial phosphoric acid produced by hydrochloric acid method
An industrial phosphoric acid and hydrochloric acid method technology, applied in chemical instruments and methods, phosphorus compounds, inorganic chemistry and other directions, can solve the problems of being unfavorable to large-scale industrial production, unrealized industrial production, low extraction efficiency, etc., and achieves moderate boiling point, Recycling is convenient and the effect of saving solvent loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Add hydrochloric acid with a mass concentration of about 21% into the acid hydrolysis tank, add phosphate rock powder under stirring, and the amount of hydrochloric acid is 1.2 times the theoretical amount of hydrochloric acid required when the CaO in the phosphate rock powder is completely reacted with hydrochloric acid. The reaction was carried out at 50° C. under vigorous stirring for 2 hours, and after completion of the reaction, the solution was filtered to obtain an acid hydrolyzed solution.
[0027] Concentrate the clarified acid hydrolysate to H 3 PO 4 The mass fraction is about 20%, adding an appropriate amount of sodium carbonate for defluorination, reacting at 70 ° C for 2 hours, sedimentation, taking the supernatant liquid, adding activated carbon to decolorize, and filtering to obtain a crudely purified phosphoric acid solution.
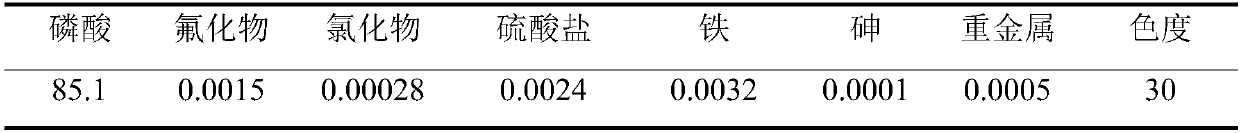
[0028] The above-mentioned crude and purified phosphoric acid solution is added to the extraction agent of the present inventio...
Embodiment 2
[0035] Add hydrochloric acid with a mass concentration of about 21% into the acid hydrolysis tank, add phosphate rock powder under stirring, and the amount of hydrochloric acid is 1.1 times the theoretical amount of hydrochloric acid required when the CaO in the phosphate rock powder is completely reacted with hydrochloric acid. The reaction was carried out at 60° C. under vigorous stirring for 0.5 hours, and after the reaction was completed, the solution was filtered to obtain an acid hydrolyzed solution.
[0036] Concentrate the clarified acid hydrolysate to H 3 PO 4 The mass fraction is about 25%, adding an appropriate amount of sodium carbonate for defluorination, reacting at 70 ° C for 2 hours, sedimentation, taking the supernatant liquid, adding activated carbon to decolorize, and filtering to obtain a crudely purified phosphoric acid solution.
[0037] The above-mentioned crude and purified phosphoric acid solution is added to the extractant of the present invention in...
Embodiment 3
[0044] Add hydrochloric acid with a mass concentration of about 21% into the acid hydrolysis tank, add phosphate rock powder under stirring, and the amount of hydrochloric acid is 1.05 times the theoretical amount of hydrochloric acid required when the CaO in the phosphate rock powder is completely reacted with hydrochloric acid. The reaction was carried out at 50° C. under vigorous stirring for 2 hours, and after completion of the reaction, the solution was filtered to obtain an acid hydrolyzed solution.
[0045] Concentrate the clarified acid hydrolysate to H 3 PO 4 The mass fraction is about 20%, adding an appropriate amount of sodium carbonate for defluorination, reacting at 70 ° C for 2 hours, sedimentation, taking the supernatant liquid, adding activated carbon to decolorize, and filtering to obtain a crudely purified phosphoric acid solution.
[0046] The above-mentioned crude and purified phosphoric acid solution is added to the extractant of the present invention in ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com