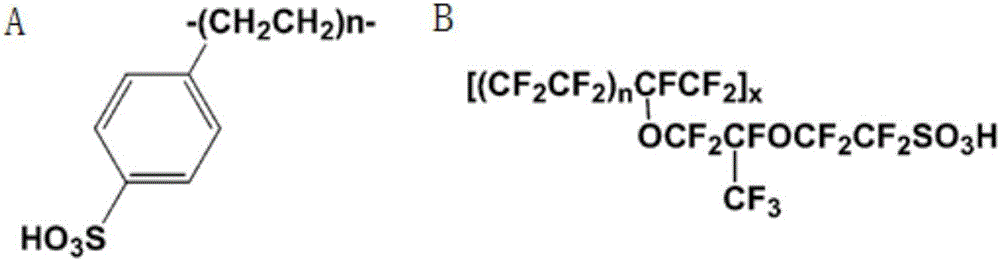2-branch acylation method of solid acid continuous loaded catalyzed aromatic heterocyclic compound
A technology of solid acid catalysts and compounds, applied in the direction of organic compound/hydride/coordination complex catalysts, chemical instruments and methods, catalytic reactions, etc., can solve problems such as unfavorable industrial scale-up application, high reaction temperature, and insufficient economy , to achieve considerable economic benefits, good reaction selectivity, and easy separation of products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1: the screening of catalyst
[0039]
[0040] Catalyst to be screened is as shown in table 1, and this screening obtains by batch reaction, and its concrete operation is as follows:
[0041] At room temperature, 1-toluenesulfonyl-1H-pyrrole (10mmol, 1.0eq) was added to a three-necked flask, and an appropriate amount of DCM (50mL) was added to dissolve it, and then acetic anhydride (20mmol, 2.0eq) was slowly added, stirred for 15min, and once Add solid acid (100wt%). The above mixture was stirred at room temperature, monitored by TLC, until the starting material was consumed.
[0042] Table 1 shows the screening results. It can be seen from the experimental results that solid acid and traditional Lewis acid such as AlCl 3 Compared with, has a very different regioselectivity: with AlCl 3 Catalysis can efficiently obtain 3-acetylated products, while solid acid catalysis mainly obtains 2-acetylated products. And among solid acids, H-β zeolite has the best...
Embodiment 2
[0051] Example 2: Continuous preparation of 1-(1-tosyl-1H-pyrrol-2-yl)ethanone
[0052]
[0053] Put the H-β zeolite in a crucible, bake it in a muffle furnace at 500-600°C for 4 hours, cool it down to room temperature, and use it now or keep it sealed.
[0054] The activated H-beta zeolite (500wt%) was placed as attached Figure 4 In the kettle body 3 of the shown fixed-bed reactor, after the tightness detection, start to feed, and pump in N-Ts-pyrrole (2.21g, 2.21g, 10mmol, 1.0eq) and acetic anhydride (1.12g, 11mmol, 1.1eq) dissolved in dichloromethane (10mL), the temperature of circulating water in the jacket was 65°C, and the feeding speed was 1.0mL / min (by adjusting the feeding pump 4 The flow rate to control the reaction time of the reaction solution in the continuous reaction device). The reaction liquid flowing out from the kettle body 3 was collected by the receiver 1, and the feeding was completed after 1 hour. The system in the receiving bottle was detected by ...
Embodiment 3
[0056] Example 3: Continuous preparation of 1-(1-tosyl-1H-pyrrol-2-yl)ethanone
[0057] Catalyst Amberlyst-15 (500wt%) is placed as attached Figure 4 In the kettle body 3 of the shown fixed-bed reactor, after the tightness detection, start to feed, and pump in N-Ts-pyrrole (2.21g, 2.21g, 10mmol, 1.0eq) and acetic anhydride (1.12g, 11mmol, 1.1eq) dissolved in dichloromethane (10mL), the temperature of circulating water in the jacket was 65°C, and the feeding speed was 1.0mL / min. The reaction liquid flowing out from the kettle body 3 was collected by the receiver 1, and the feeding was completed after 1 hour. The system in the receiving bottle was detected by HPLC. The raw material was completely converted, the 2-acetylation selectivity was 97%, and the product purity was 93%. The solution in the receiving bottle was directly concentrated to dryness to obtain the target product with a yield of 95%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com