Preparation method of magnetic iron oxide
A soluble, ferrous salt technology, applied in the direction of iron oxide, iron oxide/iron hydroxide, nitrogen fertilizer, etc., can solve the problems of reduced active sites, reduced specific surface area, and fewer active sites, so as to improve the penetration sulfur capacity , many crystal defects, and large specific surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
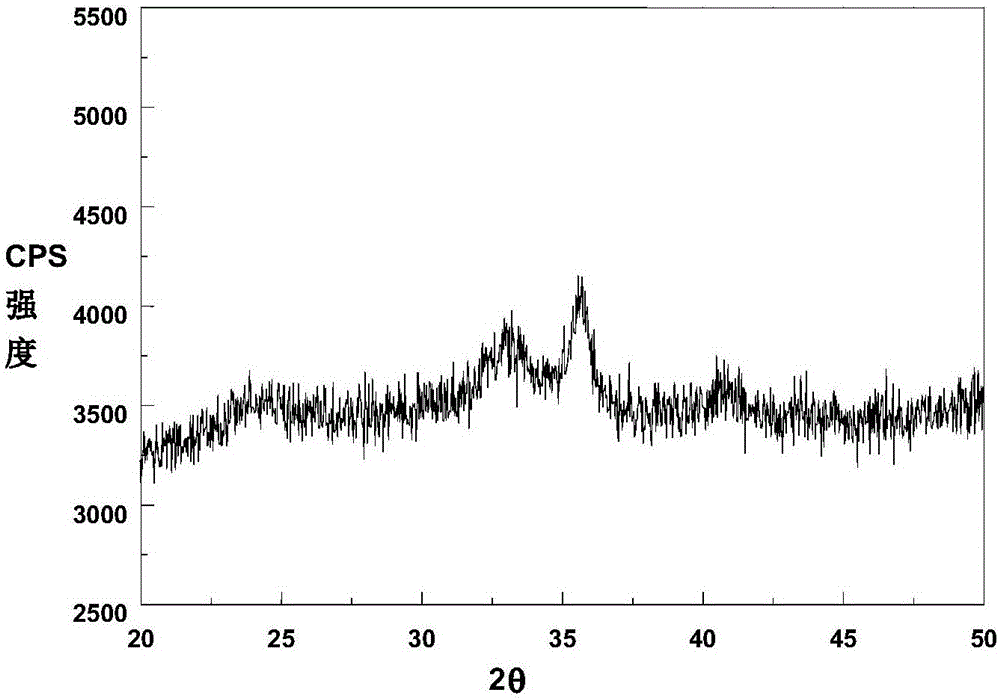
Image
Examples
Embodiment 1
[0029] This embodiment provides a preparation method for preparing high specific surface magnetic iron oxide, comprising the following steps:
[0030] 1) Mix ferrous sulfate heptahydrate solid and ammonium carbonate solid with a molar ratio of 1:2.4 at 25°C, and add a certain amount of water to form a slurry during the kneading process, and control the pH of the slurry during the kneading process The value is 6.5-6.8, and the pH value of the slurry is 7.5-8 at the end of kneading;
[0031] 2) performing the first solid-liquid separation on the slurry obtained in step 1), collecting the first solid phase and the first liquid phase, and washing the first solid phase with water, collecting the washing liquid, and dissolving the first liquid phase Phase and the washing liquid are evaporated to dryness to obtain ammonium sulfate;
[0032] 3) using water to make the solid phase into a slurry with a concentration of 15wt%, adding a concentration of 30wt% hydrogen peroxide to the slu...
Embodiment 2
[0037] This embodiment provides a preparation method for preparing high specific surface magnetic iron oxide, comprising the following steps:
[0038] 1) Knead ferrous sulfate heptahydrate solid and ammonium bicarbonate solid with a molar ratio of 1:4 at 20°C, and add a certain amount of water to form a slurry during the kneading process, and control the amount of the slurry during the kneading process. The pH value is 6.8-7, and the pH value of the slurry at the end of the kneading is 7-7.5;
[0039] 2) performing the first solid-liquid separation on the slurry obtained in step 1), collecting the first solid phase and the first liquid phase, and washing the first solid phase with water, collecting the washing liquid, and dissolving the first liquid phase Phase and the washing liquid are evaporated to dryness to obtain ammonium sulfate;
[0040] 3) using water to make the solid phase into a slurry with a concentration of 20wt%, adding a concentration of 40wt% hydrogen peroxide ...
Embodiment 3
[0044] This embodiment provides a preparation method for preparing high specific surface magnetic iron oxide, comprising the following steps:
[0045] 1) Mix ferrous sulfate heptahydrate solid and ammonium carbonate solid with a molar ratio of 1:3 at 30°C, and add a certain amount of water to form a slurry during the kneading process, and control the pH of the slurry during the kneading process The value is 6.5-6.8, and the pH value of the slurry is 7.5-8 at the end of kneading;
[0046] 2) performing the first solid-liquid separation on the slurry obtained in step 1), collecting the first solid phase and the first liquid phase, and washing the first solid phase with water, collecting the washing liquid, and dissolving the first liquid phase Phase and the washing liquid are evaporated to dryness to obtain ammonium sulfate;
[0047] 3) using water to make the solid phase into a slurry with a concentration of 10wt%, adding a concentration of 50wt% hydrogen peroxide to the slurr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com