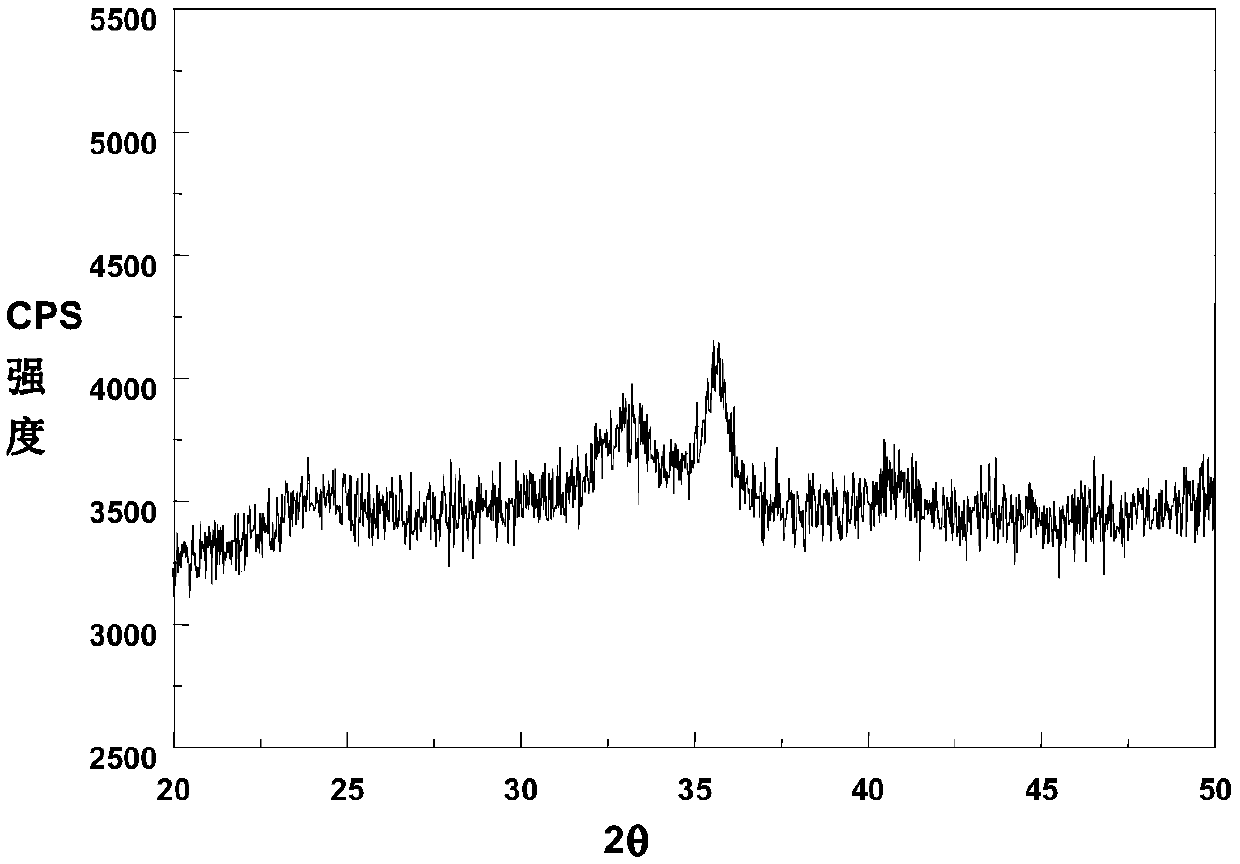
A kind of preparation method of magnetic iron oxide
A technology of iron oxyhydroxide and hydrogen peroxide, applied in chemical instruments and methods, oxidized water/sewage treatment, ammonium sulfate, etc., can solve the problem of physical adsorption capacity and chemical adsorption capacity decline, specific surface area reduction, active site reduction, etc. problems, achieve the effects of shortening the preparation cycle, large specific surface area, and improving the breakthrough sulfur capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] This embodiment provides a method for preparing magnetic iron oxide, comprising the following steps:
[0038] 1) adding sulfuric acid with a concentration of 60wt% to the ferrous sulfate heptahydrate solid, stirring evenly to make it fully acidified, wherein the molar ratio of ferrous sulfate to sulfuric acid is 1:0.25;
[0039] 2) Mix the acidified ferrous sulfate with 50wt% hydrogen peroxide and react at 20°C for 0.5h to prepare an iron oxyhydroxide precursor, wherein the molar ratio of the ferrous sulfate to the hydrogen peroxide is 1: 0.7;
[0040] 3) neutralizing the iron oxyhydroxide precursor and ammonium bicarbonate at a molar ratio of 1:5 at 25° C. for 0.5 h to obtain a reaction product;
[0041] 4) performing solid-liquid separation on the reaction product, collecting the solid phase and the liquid phase respectively, evaporating the liquid phase to dryness to obtain ammonium sulfate, and washing the solid phase with water;
[0042] 5) First prepare the solid ...
Embodiment 2
[0047] This embodiment provides a method for preparing magnetic iron oxide, comprising the following steps:
[0048] 1) adding sulfuric acid with a concentration of 70wt% to the ferrous sulfate heptahydrate solid, stirring evenly to make it fully acidified, wherein the molar ratio of ferrous sulfate to sulfuric acid is 1:0.4;
[0049] 2) Mix the acidified ferrous sulfate with 40wt% hydrogen peroxide and react at 30°C for 1 hour to prepare an iron oxyhydroxide precursor, wherein the molar ratio of the ferrous sulfate to the hydrogen peroxide is 1:0.6 ;
[0050] 3) neutralizing the iron oxyhydroxide precursor and ammonium carbonate at a molar ratio of 1:7 at 50° C. for 1 hour to obtain a reaction product;
[0051] 4) performing solid-liquid separation on the reaction product, collecting the solid phase and the liquid phase respectively, evaporating the liquid phase to dryness to obtain ammonium sulfate, and washing the solid phase with water;
[0052] 5) First prepare the soli...
Embodiment 3
[0057] This embodiment provides a method for preparing magnetic iron oxide, comprising the following steps:
[0058] 1) adding sulfuric acid with a concentration of 80wt% to the ferrous sulfate heptahydrate solid, stirring evenly to make it fully acidified, wherein the molar ratio of ferrous sulfate to sulfuric acid is 1:0.3;
[0059] 2) Mix the acidified ferrous sulfate with 30wt% hydrogen peroxide and react at 25° C. for 1.5 h to prepare an iron oxyhydroxide precursor, wherein the molar ratio of the ferrous sulfate to the hydrogen peroxide is 1: 0.55;
[0060] 3) neutralizing the iron oxyhydroxide precursor and ammonium bicarbonate at a molar ratio of 1:5 at 25° C. for 1.5 h to obtain a reaction product;
[0061] 4) performing solid-liquid separation on the reaction product, collecting the solid phase and the liquid phase respectively, evaporating the liquid phase to dryness to obtain ammonium sulfate, and washing the solid phase with water;
[0062] 5) Prepare the washed ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com