Method for preparing phenolic compounds by catalytic conversion of acetone with ceria
A technology for catalyzing acetone and phenolic compounds with ceria, which is applied in the preparation of organic compounds, chemical instruments and methods, organic chemistry and other directions, can solve the problems of reducing the selectivity and difficulty of phenolic compounds, and achieves high efficiency and simple preparation. , the effect of high reaction conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
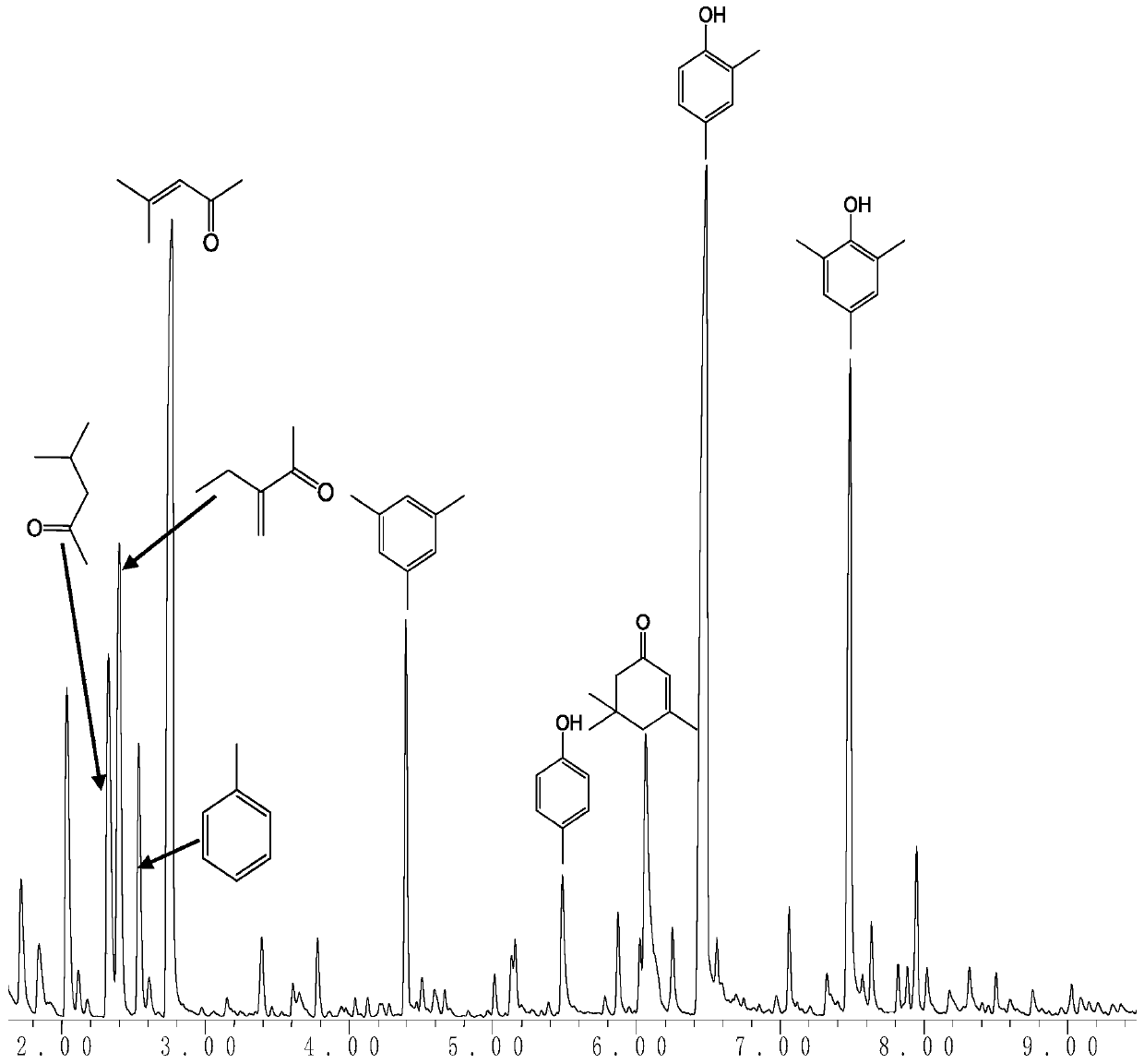
Image
Examples
Embodiment 1
[0026] Weigh 200g of ammonium cerium nitrate and bake it at 500°C for 5h under air atmosphere to obtain cerium dioxide. Fill the catalyst with 14-25 mesh of ceria forming sieve into a reaction tube with an inner diameter of 7mm, and fill it with a 15cm bed. Under normal pressure, the feed rate of acetone is 0.03mL·min -1 , nitrogen as the carrier gas, the flow rate is 30mL·min -1 , Reacted at 350°C for 12h, after the reaction was completed, the liquid was separated and analyzed by chromatography. The conversion of acetone was 65%, and the overall selectivity to phenolic compounds was 35%.
Embodiment 2
[0028] Weigh 200g of ammonium cerium nitrate and bake it at 500°C for 5h under air atmosphere to obtain cerium dioxide. Fill the catalyst with 14-25 mesh of ceria forming sieve into a reaction tube with an inner diameter of 8mm, fill it with a 25cm bed, and under normal pressure, the feed rate of acetone is 0.03mL·min -1 , nitrogen as the carrier gas, the flow rate is 30mL·min -1 , reacted at 350°C for 12h, and analyzed by chromatography after the reaction. The conversion of acetone was 58%, and the overall selectivity to phenolic compounds was 44%.
Embodiment 3
[0030] Weigh 200g of ammonium cerium nitrate and bake it at 500°C for 5h under air atmosphere to obtain cerium dioxide. Fill the catalyst with 14-25 mesh of ceria forming sieve into a reaction tube with an inner diameter of 10mm, fill it with a 30cm bed, and under normal pressure, the feed rate of acetone is 0.03mL·min -1 , nitrogen as the carrier gas, the flow rate is 30mL·min -1 , reacted at 350°C for 12h, and analyzed by chromatography after the reaction. The conversion of acetone was 68%, and the overall selectivity to phenolic compounds was 33%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com