Method for culturing and inducing tilapia mossambica peritoneal preadipocytes and culture medium thereof
A technology for inducing culture medium and preadipocytes, which is applied in the cultivation process, animal cells, tissue culture, etc., to achieve the effect of broad application prospects, convenient promotion and application, and realization of in vitro subculture
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] The present embodiment is exemplified by "in vitro extraction and cultivation of tilapia (Tilapia) abdominal adipocytes"
[0080] 1. Experimental animals:
[0081] Tilapia (700-900g) that has just died (it can also be purchased from the market, is healthy and has no body surface damage)
[0082] 2. Experimental steps:
[0083] (1) Bleeding by cutting off the gill arch (if the tilapia is healthy, the tilapia should be anesthetized with MS-222);
[0084] (2) Under sterile conditions, quickly separate the adipose tissue around the mesentery, put it directly into the Krebs-Henseleit buffer, wash it three times, cut it into pieces and place it in a centrifuge tube, then add an equal volume of 1% type II collagenase to the 28 ℃ water bath shaking digestion for 1h;
[0085] (3) filter the suspended fat cells with 100 mesh nylon membrane, remove connective tissue, and wash three times;
[0086] (4) Add erythrocyte lysate to remove blood cells, resuspend cells in DMEM / F12 me...
Embodiment 2
[0100]Experimental steps (1)~(3) are with embodiment 1;
[0101] In step (4), red blood cell lysate was added to remove blood cells, cells were resuspended in DMEM / F12 medium containing fetal bovine serum, and seeded in cell plates at 28°C, 5% CO 2 Cultivate under conditions, change the medium every 4 days, and cultivate for 14 to 20 days;
[0102] (5) The cultured preadipocytes were digested with 0.12% trypsin, resuspended, and mixed with 2~3×10 5 cells / ml inoculated in the proliferation medium, cultured for 2-3 days, the cells can cover the cell plate.
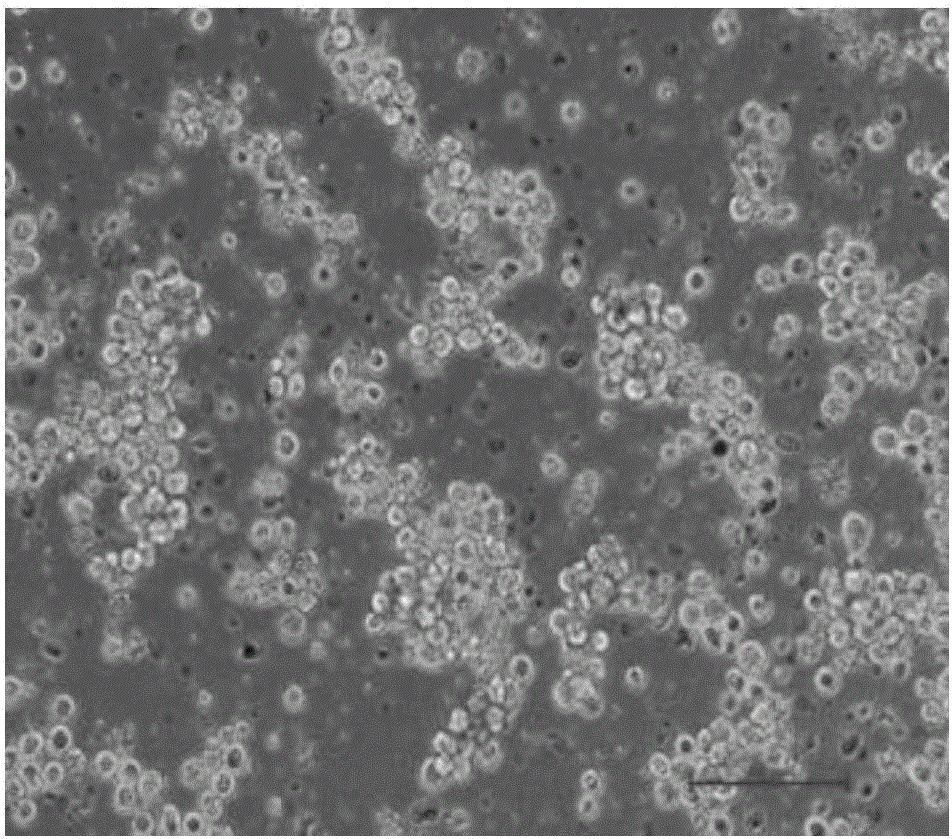
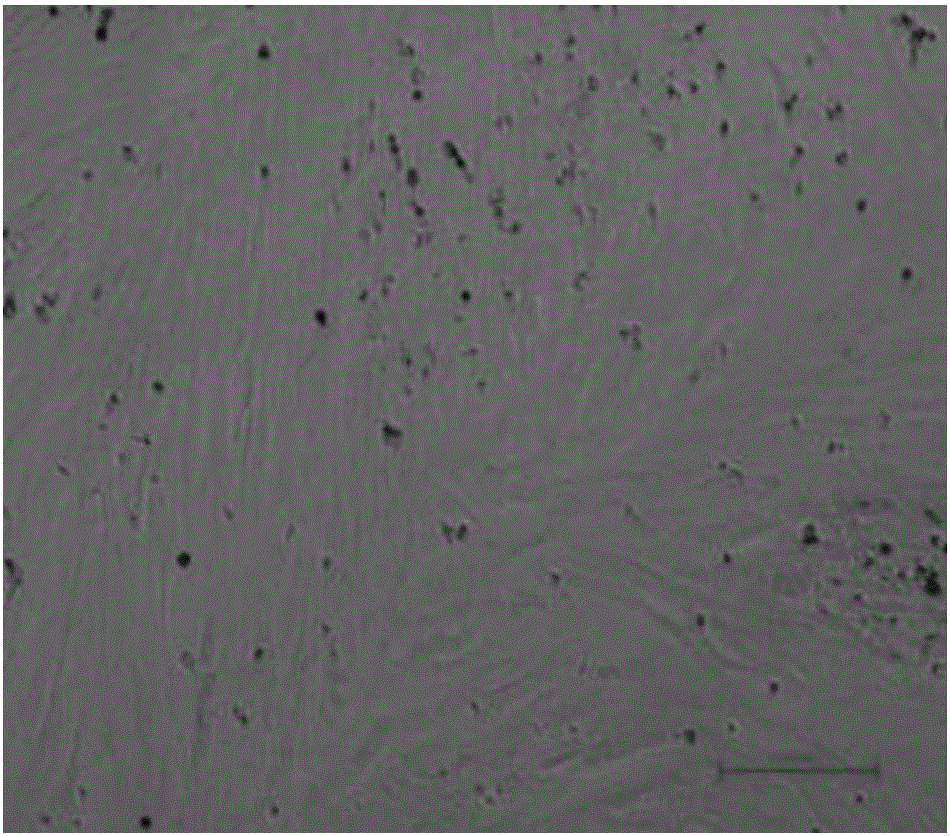
[0103] In this embodiment, the cells that have been successfully passaged can continue to proliferate, and can cover the entire bottom of the cell plate in 2 to 3 days.
[0104] Figure 5 In order to pass the cells cultured in step (4) to a new cell plate, use 2~3×10 5 cells / ml inoculated, cultured for 2-3 days, the cells can cover the bottom of the entire cell plate.
Embodiment 3
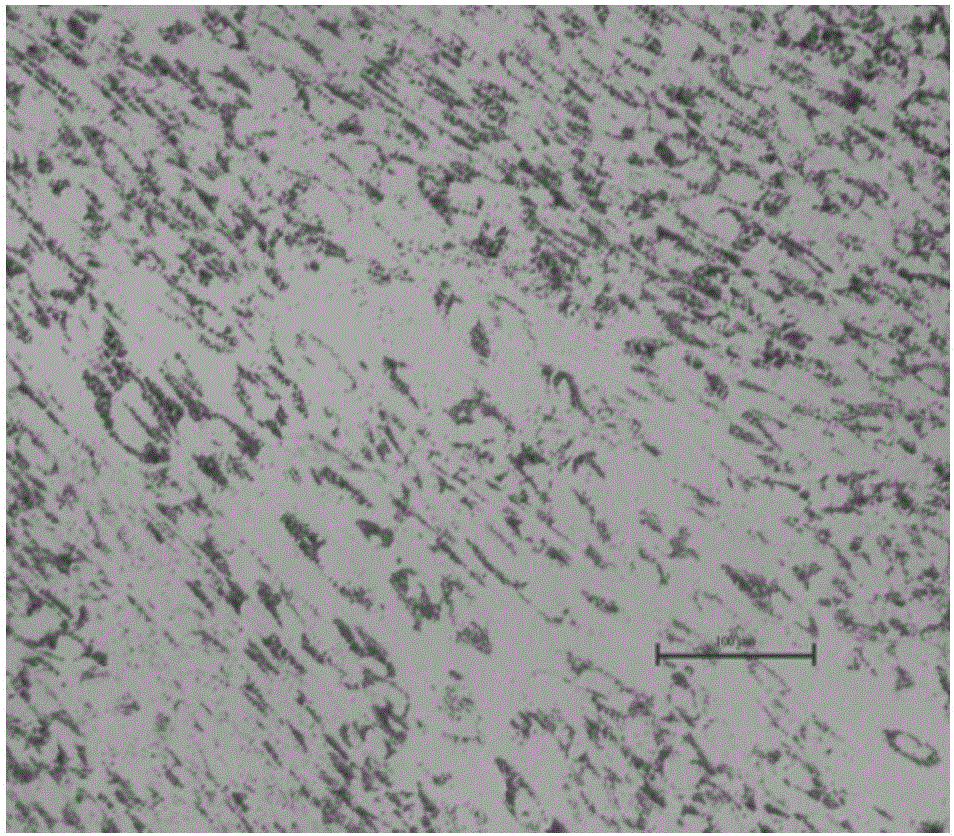
[0106] Other conditions are the same as in Example 1. When the induction medium in steps (4) to (5) is the grass carp induction medium, the lipid droplets produced by the cells are not obvious, such as Figure 6 The figure shows that when the grass carp induction medium was induced for 12 days, the oil red staining showed that the lipid droplets in the cells were blurred, and almost no clear lipid droplets were produced in the cells. Neutral fat cells almost disappeared.
[0107] The composition of the grass carp induction medium is DMEM / F12 medium with 15% fetal bovine serum, 20 μg / ml insulin, 1 μmol / L dexamethasone, and 10 nmol / L triiodothyronine.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com