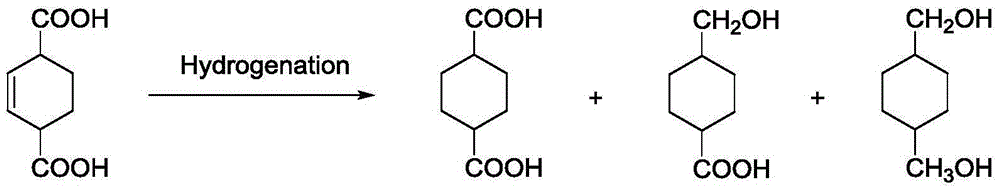
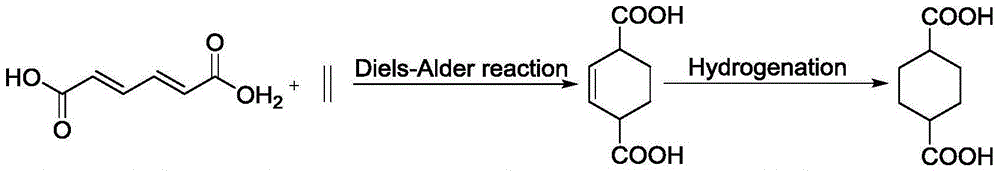Preparation method for 1,4-cyclohexane dicarboxylic acid (CHDA) and diester thereof
A technology of cyclohexanedicarboxylic acid diester and cyclohexanedicarboxylic acid, which is applied in the field of chemical engineering or polymer science to achieve the effect of reducing dependence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
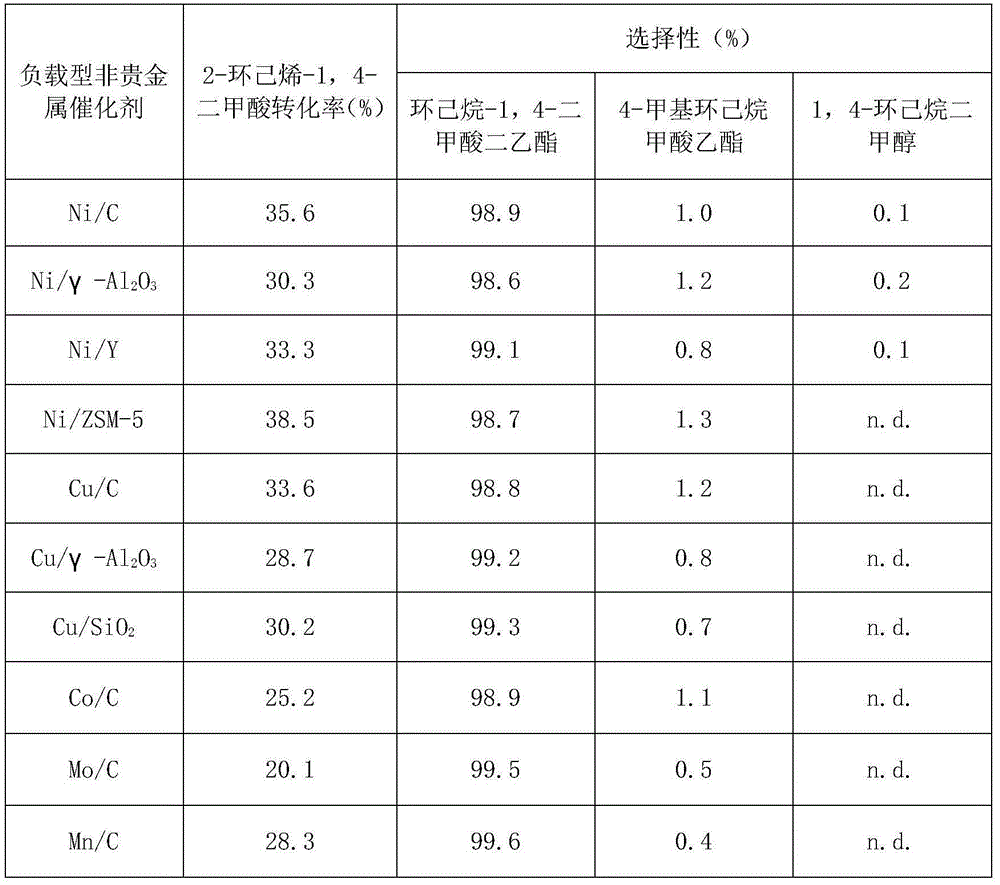
[0025] Example 1 Performance Evaluation of Supported Non-noble Metal Catalysts for Hydrogenation of 2-Cyclohexene-1,4-Dicarboxylic Acid
[0026] In this example, the performance of the supported non-noble metal catalyst in catalyzing the dehydrogenation reaction of 2-cyclohexene-1,4-dicarboxylic acid was studied.
[0027] In an autoclave with a polytetrafluoro liner, add 1.58g 2-cyclohexene-1,4-dicarboxylic acid and 15.8g of solvent ethanol, wherein 2-cyclohexene-1,4-dicarboxylic acid and The mass ratio of ethanol is 1:10, and the molar ratio of the metal active component of the catalyst used to 2-cyclohexene-1,4-dicarboxylic acid is 1:100. After stirring and mixing at room temperature, the 5MPa nitrogen replacement reactor 5 For the second time, 1.0 MPa hydrogen gas was introduced, the temperature was raised to 80° C. by electric heating, and the reaction was performed for 4 hours under magnetic stirring at 1000 rpm. Stop stirring, cool the reaction kettle to room temperatur...
Embodiment 2
[0030]Example 2 Performance evaluation of supported noble metal catalysts for dehydrogenation of 2-cyclohexene-1,4-dicarboxylic acid
[0031] In this example, the performance of the supported noble metal catalyst for the hydrogenation reaction of 2-cyclohexene-1,4-dicarboxylic acid was studied.
[0032] In an autoclave with a Teflon liner, add 1.58g of 2-cyclohexene-1,4-dicarboxylic acid and 15.8g of solvent ethanol, 2-cyclohexene-1,4-dicarboxylic acid and ethanol The mass ratio is 1:10, and the molar ratio of the metal active component of the catalyst used to 2-cyclohexene-1,4-dicarboxylic acid is 1:100. After stirring and mixing at room temperature, replace the reactor with 5MPa nitrogen for 5 times , 1.0 MPa hydrogen gas was introduced, the temperature was raised to 80° C. by electric heating, and the reaction was carried out for 4 hours under magnetic stirring at 1000 rpm. Stop stirring, cool the reaction kettle to room temperature with ice water, take an appropriate amou...
Embodiment 3
[0035] Example 3 The influence of solvent on the hydrogenation reaction of 2-cyclohexene-1,4-dicarboxylic acid
[0036] This example studies the influence of different polar and non-polar solvents on the hydrogenation reaction of 2-cyclohexene-1,4-dicarboxylic acid.
[0037] Add the 2-cyclohexene-1,4-dicarboxylic acid reaction solution with a mass fraction of 10% into a high-pressure reactor with a polytetrafluoroethylene liner, and the reaction solution is composed of 2-cyclohexene-1,4-dicarboxylic acid Composition of formic acid and the solvent described in Table 3, adding a Ru / C catalyst whose metal active component is 1 / 100 of the molar weight of 2-cyclohexene-1,4-dicarboxylic acid, stirring and mixing at room temperature, and replacing with 5MPa nitrogen The reactor was fed 5 times with 1.0MPa hydrogen gas, heated to 80°C by electric heating, and reacted for 4 hours under 1000rpm magnetic stirring. Adopt the method of embodiment 1 to carry out qualitative and quantitativ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com