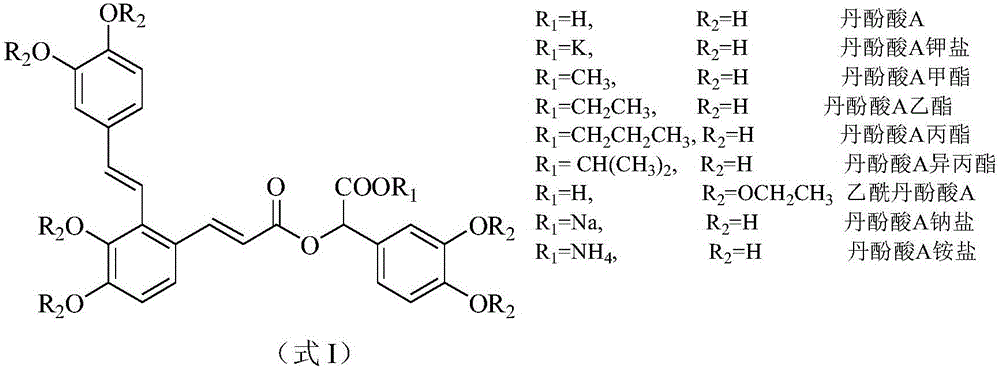
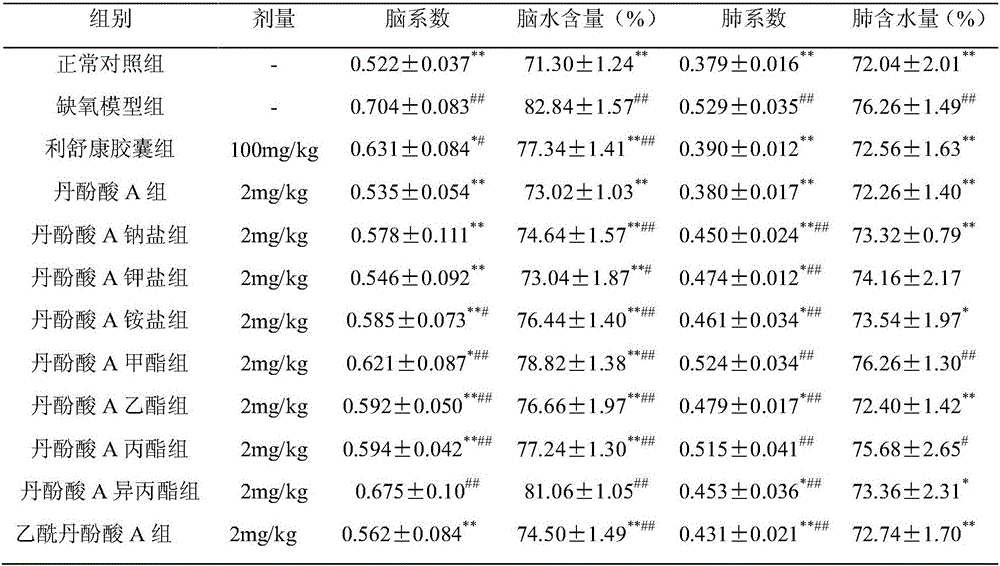
Application of salvianolic acid A and derivatives thereof to prevention and treatment of high-altitude sickness
A technology of salvianolic acid and derivatives, applied in the field of medicine, can solve the problems of low absorption efficiency, high urine output, dehydration, slow onset of action and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Salvianolic acid A powder injection:
[0036]
[0037] Add salvianolic acid A, mannitol, and sodium thiosulfate to 800ml of water for injection according to the prescription, adjust the pH to 4-6 with 0.5% sodium hydroxide; stir well with 0.1% activated carbon for injection, keep warm at 60°C for 30 minutes, Filtrate and decarbonize while it is hot, add water for injection to the filtrate to 1000ml, sterilize and fine filter, fill in 200 10ml vials, half stoppered, send to a freeze dryer, pre-freeze at -40°C for 2 hours, and vacuum to 10Pa~20Pa, Incubate at -25°C for 3h, at -10°C for 16h, dry at 30°C for 3h, press the plug out of the box, and obtain salvianolic acid A powder injection.
Embodiment 2
[0039] Salvianolic Acid Ethyl Ester Tablets:
[0040]
[0041] Grind ethyl salvianolic acid A through a 80-mesh sieve, weigh the sieved ethyl salvianolic acid A, spray-dried lactose, crospovidone, and mix evenly with sodium bisulfite, then add the 60-mesh sieved Mix micronized silica gel, and finally add magnesium stearate to mix evenly, and make 7mm round shallow concave punching tablets. Dissolve the gastric-soluble coating premix in purified water to make a coating solution with a solid content of 15%, and use a high-efficiency coating machine for coating, and the weight of the coating will increase by 3%, so as to obtain ethyl salvianolic acid A Coated tablets (based on salvianolic acid A, 60mg / tablet).
Embodiment 3
[0043] Salvianolic acid A sodium salt capsules:
[0044]
[0045] Grind the salvianolic acid A sodium salt and vitamin C, pass through an 80-mesh sieve, weigh the crushed and sieved salvianolic acid A sodium salt, vitamin C, microcrystalline cellulose, and pregelatinized starch, mix them well, and add Appropriate amount of water and ethanol is made into a soft material, granulated with a 20-mesh sieve, dried at 40°C, the dry granules are sorted with a 20-mesh sieve, added with croscarmellose sodium and magnesium stearate and mixed evenly to obtain a total blended medicinal powder. The total mixed medicinal powder is filled in 2# hollow capsules, with a grain weight of 170 mg, to obtain salvianolic acid A sodium salt capsules (calculated as salvianolic acid A, 60 mg / capsule).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com