Application of bone morphogenetic protein-9 in preparing pharmaceutical composition for treating and/or preventing cholinergic system degeneration related diseases
A cholinergic and composition technology, applied in the field of bone morphogenetic protein-9, can solve problems such as the application of bone morphogenetic protein-9 that has not yet been reported, and achieve the effects of avoiding first-pass effect, improving memory and cognitive ability, and being easy to popularize
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0031] Experimental example 1 Establishment of a mouse model of cholinergic neuron (BFCS) degeneration
[0032] Select 40 12-week-old male C57 mice (inbred mice) and randomly divide them into control group (Con), thiamine-deficient group (PTD), BMP-9 group, thiamine-deficient + BMP-9 group (PTD+BMP-9), 10 mice in each group, all animals were kept in SPF (Specific Pathogen Free) grade room at room temperature 25°C between light and dark for 12h, free to eat and drink.
[0033] The control group was given normal diet + intraperitoneal injection of normal saline 5 μL / (g.d); the thiamine-deficient group was given thiamine-deficient feed + intraperitoneal injection of pyrithiamine 0.5 μg / (g.d); the BMP group was given normal diet + intraperitoneal injection of normal saline 5 μL / (g.d) + nasal drop of 1ng / (g.d) BMP-9; thiamine-deficient + BMP-9 group was given thiamine-deficient feed + intraperitoneal injection of pyrithiamine 0.5 μg / (g.d) + nasal drop Add 1ng / (g.d) of recombinant ...
experiment example 2
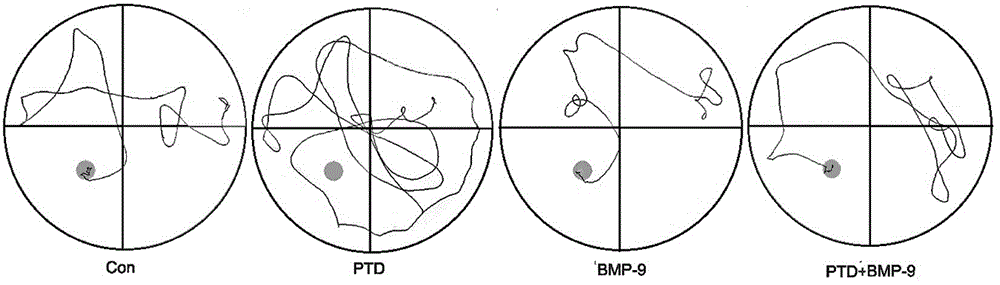
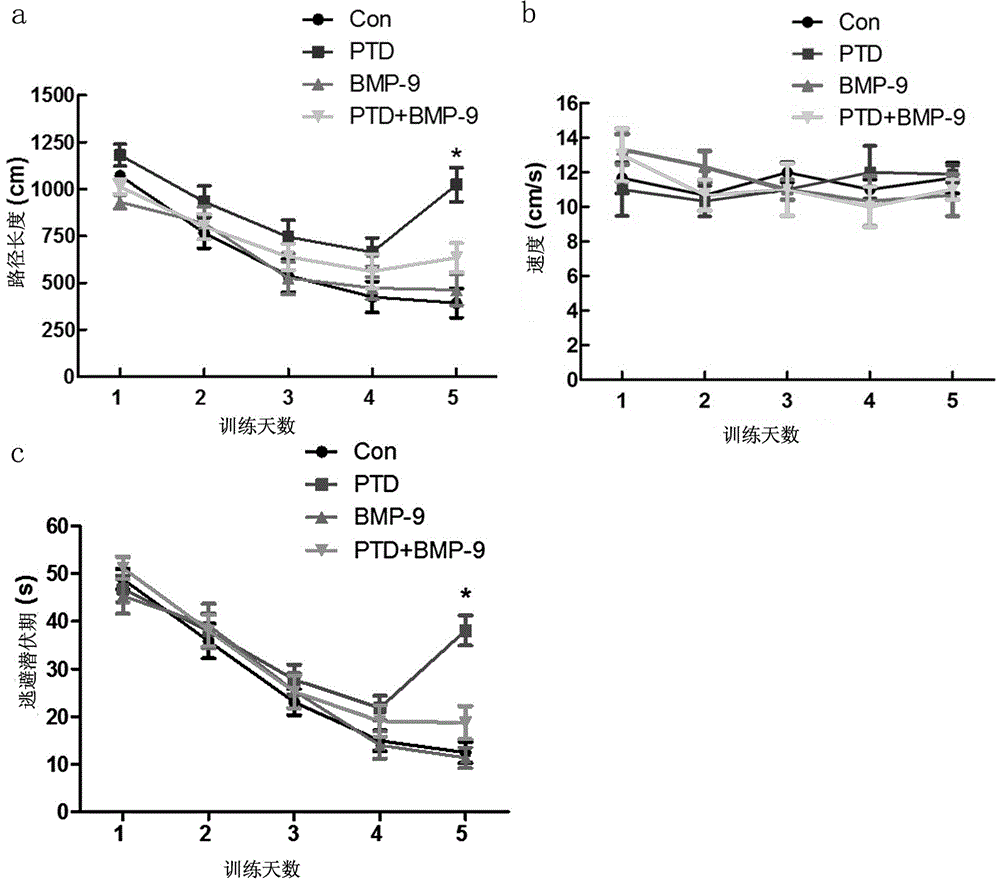
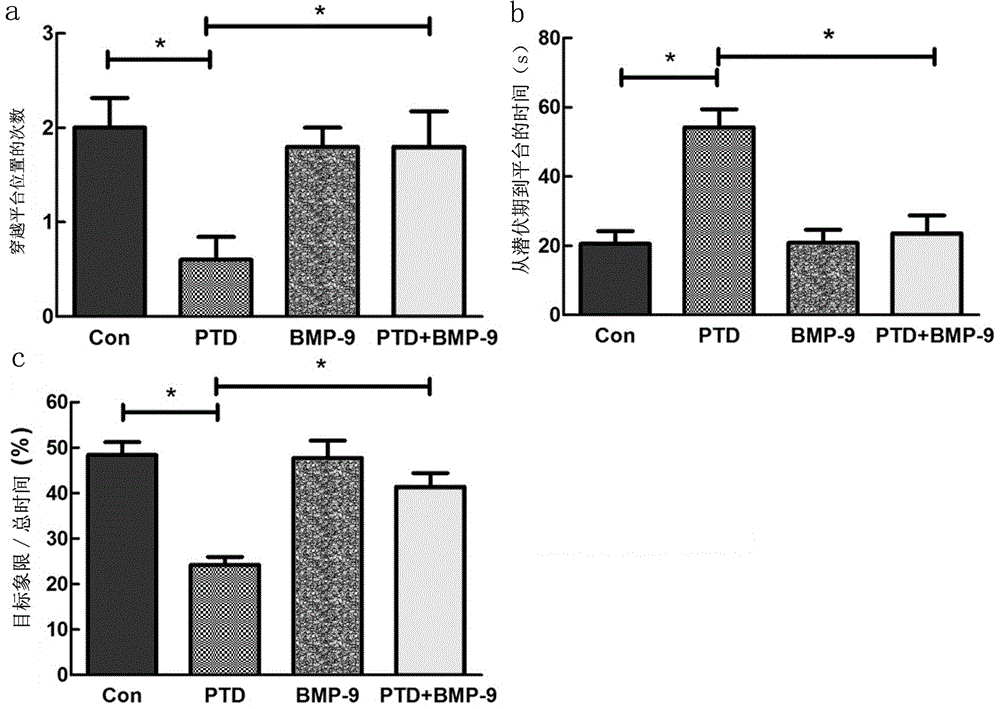
[0036] Experimental example 2 Morris water maze detection
[0037] On the 5th day after nasal administration, the Morris water maze test was started. The specific inspection methods are as follows:
[0038](1) Training stage: Put the mouse head into the water with its head facing the pool wall, and record the time when the mouse reached the underwater platform, the distance traveled to the platform and the average swimming speed. If the mouse did not find the platform within 60 s, Guide the mouse to the platform, and take it away after it stays on the platform for 20 s. If the mouse reaches the platform within 60 s, let it stay on the platform for 15 s, and then take it out. Training 8 times, each training time interval is 30 minutes, training a total of 5 days.
[0039] (2) Platform withdrawal experiment: the platform was evacuated, the space exploration training was started for 60 s, and the time for the mice to find the platform hidden under the water surface (escape laten...
experiment example 3
[0050] Experimental example 3 Immunofluorescence staining
[0051] After the mice were anesthetized, the thoracotomy was intubated to the ascending aorta, and then perfused with 60 mL of normal saline, and then fixed with 100 mL of 4% paraformaldehyde. Place in 20% and 30% sucrose solution in turn until the tissue sinks to the bottom, and after embedding in OCT (Optimal Cutting Temperature Compound, a water-soluble mixture of polyethylene glycol and polyvinyl alcohol), perform coronal sectioning on a frozen microtome, take drift Immunofluorescence staining was performed on the slices. The brain slices were rinsed with 0.01M PBS (phosphate buffered saline) and permeabilized with 0.3% Triton-X100 (polyethylene glycol octylphenyl ether) for 30 minutes, and blocked with 5% normal donkey serum for 1 hour. Add goat anti-ChAT (acetylcholine transferase) (1:200, Millipore ® ), incubated overnight at 4°C, rinsed with 0.01M PBS and added TRITC (tetramethylrhodamine isothiocyanate)-labe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com