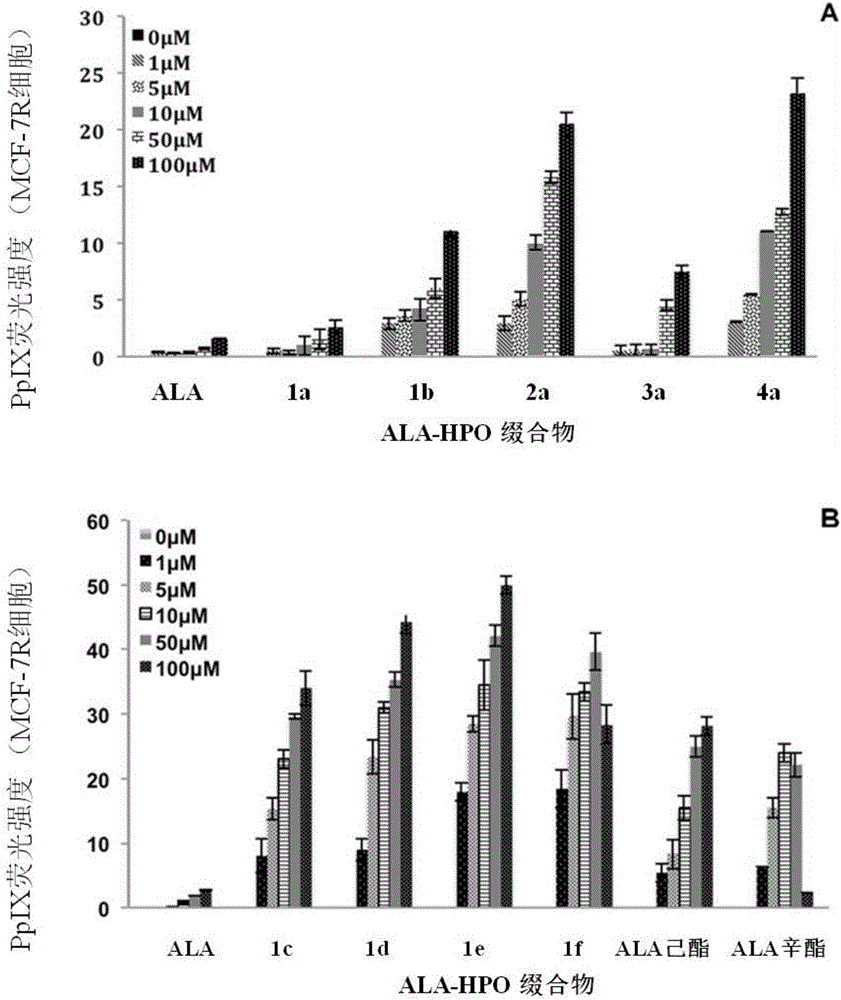
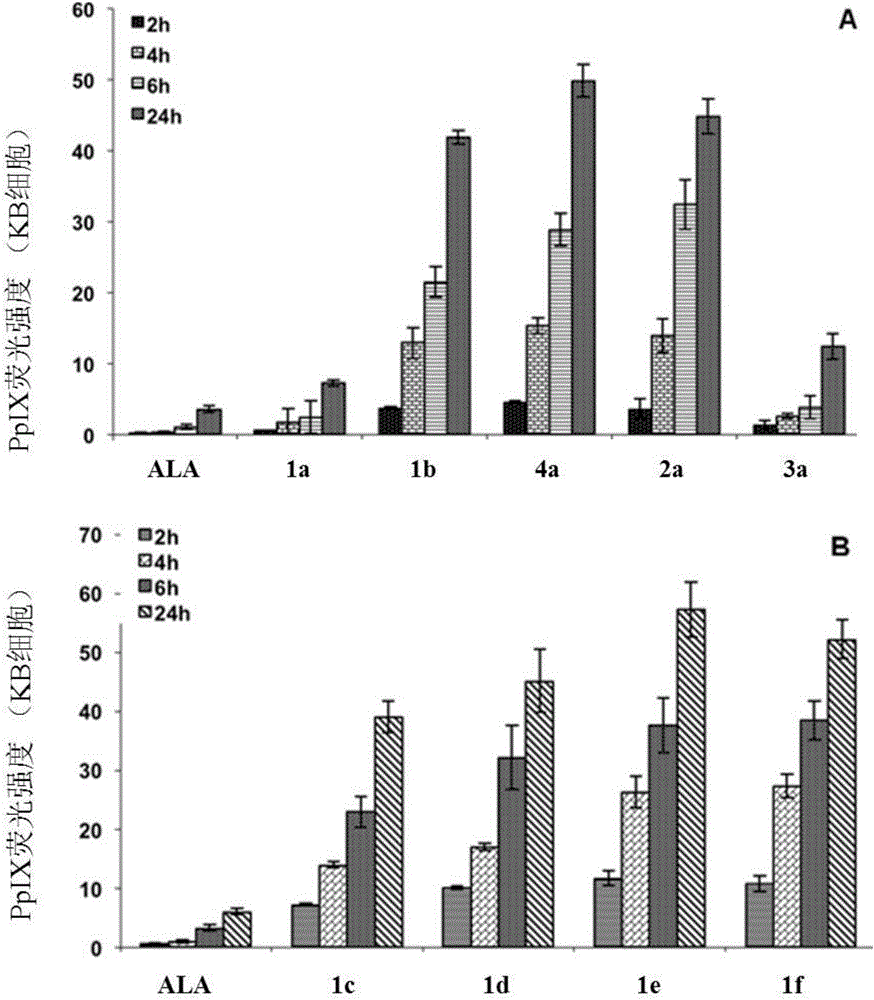
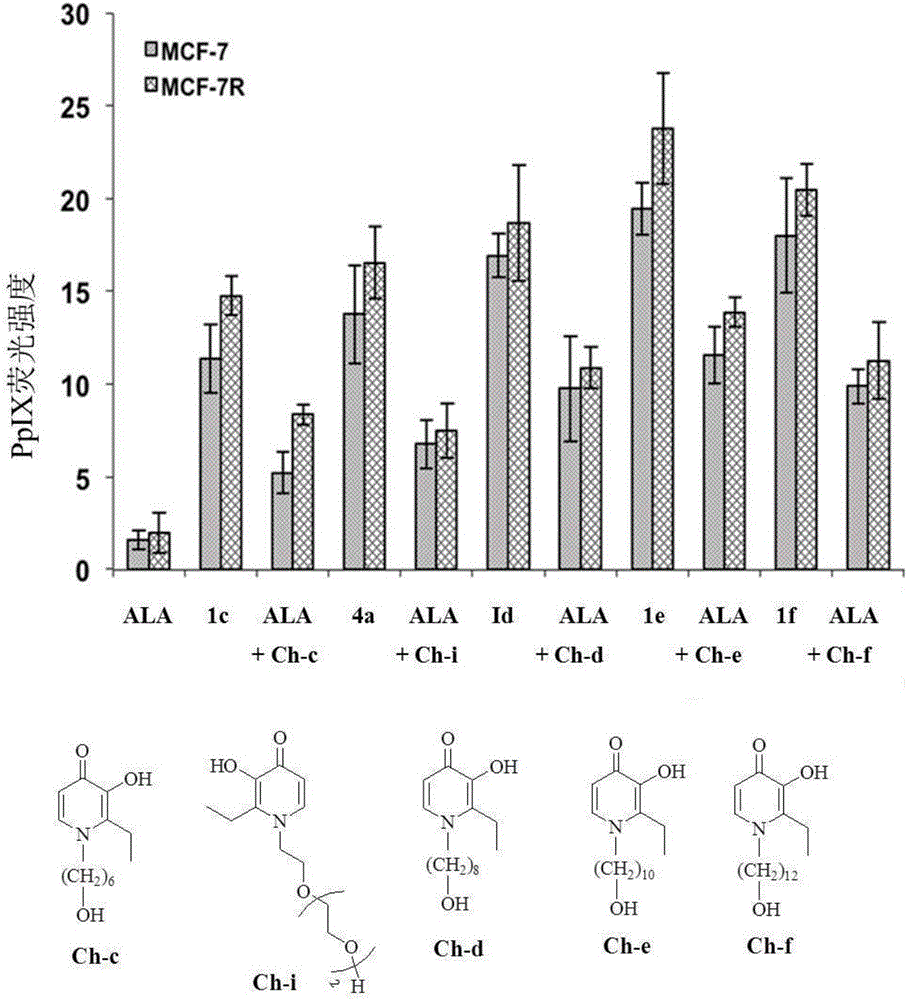
5-aminolevulinic acid/3-hydroxyl pyridone conjugate, preparation method therefor and use of 5-aminolevulinic acid/3-hydroxyl pyridone conjugate
A technology of aminolevulinic acid and hydroxypyridine, applied in 5-aminolevulinic acid-iron chelator conjugate and preparation thereof, in the field of photodynamic therapy drugs, can solve the problems of reduced drug activity and no photoactivity, etc., Achieving good biological activity and strong cellular phototoxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1, the preparation method of compound 1, carry out the following steps successively:
[0045] A, the synthesis of compound 6
[0046] Benzylation of ethyl maltol: ethyl maltol - compound 5 (84g, 0.4mol) was dissolved in methanol (300mL), NaOH solution (26.4g dissolved in 80mL H 2 O). The mixture was heated to boiling, and benzyl bromide (85.6 mL, 0.72 mol) was added dropwise over 2 hours. Dissolved and continued heating at 60-65°C overnight (12h). NaBr was removed by filtration, the filtrate was concentrated to about 1 / 3 of the original volume with a rotary evaporator), dichloromethane (400 mL) was added, and 5% (mass %) NaOH solution (3 × 250 mL), H 2 O (2×300mL) washed with anhydrous Na 2 SO 4 dry. After filtration, the obtained filtrate was evaporated to remove the solvent (mainly dichloromethane, including methanol), added diethyl ether (100 mL), and placed in a 4°C refrigerator. The product (compound 6, ie, benzyl-protected ethyl maltol) was preci...
Embodiment 2
[0076] Embodiment 2, the preparation method of compound 2a, carry out the following steps successively:
[0077] A. Synthesis of Compound 10:
[0078] Benzyl-protected ethyl maltol—a mixture of compound 9 (10g, 43.4mmol), selenium dioxide (14.5g, 130.4mmol) and bromobenzene (50mL) was vigorously stirred at 135-140°C for 4h. After cooling and filtering, the filtrate was evaporated to dryness, and the residue was chromatographically purified on a silica gel column (with about 600 g of 200-300 mesh silica gel inside) (ethyl acetate / cyclohexane, 2:1) to obtain a brown oil --- Compound 10 ( 4.6g, 43%). 1 H NMR (CDCl 3 ,400MHz): δ1.23(d,J=6.7Hz,3H,CH 3 ), 4.85(q, J=6.7Hz, 1H, CH), 5.22(d, J=2.0Hz, 2H, CH 2 ), 6.46(d, J=5.6Hz, 1H, C5-H in pyridone), 7.37(m, 5H, Ph), 7.71(d, J=5.6Hz, 1H, C6-H in pyridone).ESI -MS:m / z 247([M+H] + ).
[0079] B. Synthesis of Compound 11:
[0080] Compound 10 (4.0g, 16.2mmol) was dissolved in dichloromethane (50mL), and 50% KOH (5.45g, 48.6mmol) ...
Embodiment 3
[0090] Embodiment 3, the preparation method of compound 3a, carry out the following steps successively:
[0091] A. Synthesis of Compound 17:
[0092] Compound 16 (0.94g, 3.82mmol) and N-hydroxysuccinimide (0.485g, 4.2mmol) were dissolved in tetrahydrofuran (20ml), stirred at room temperature for 30min, DCC (0.866g, 4.2mmol) was added, and stirred for 5h. A solution of methylamine in tetrahydrofuran (2M, 4.58mmol) was added and stirred overnight at room temperature. Filtration, the filtrate was concentrated, and purified by silica gel column chromatography (containing about 150 g of 200-300 mesh silica gel) (ethyl acetate / methanol 9:1) to obtain product 17 (0.85 g, 86%). 1 H NMR (CDCl 3 ,400MHz)δ2.78(d,J=5.0Hz,3H,CH 3 ),5.39(s,2H,CH 2 ), 6.49(d, J=5.6Hz, 1H, C5-H in pyridone), 7.40(m, 5H, Ph), 7.70(br, 1H, NH), 7.84(d, J=5.6Hz, 1H, C6-H in pyridone).ESI-MS: m / z260([M+H] + ).
[0093] Note: Compound 16 was synthesized according to literature method (Xie, Y.Y.; Liu, M.S.;...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com