Fluorescent probe containing tetrasthenylvinyl group and synthesis method and application thereof
A technology of fluorescent probes and tetraphenylethylene, which is applied in the fields of fluorescence/phosphorescence, chemical instruments and methods, and the preparation of organic compounds. The method is simple, easy to modify, and the effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
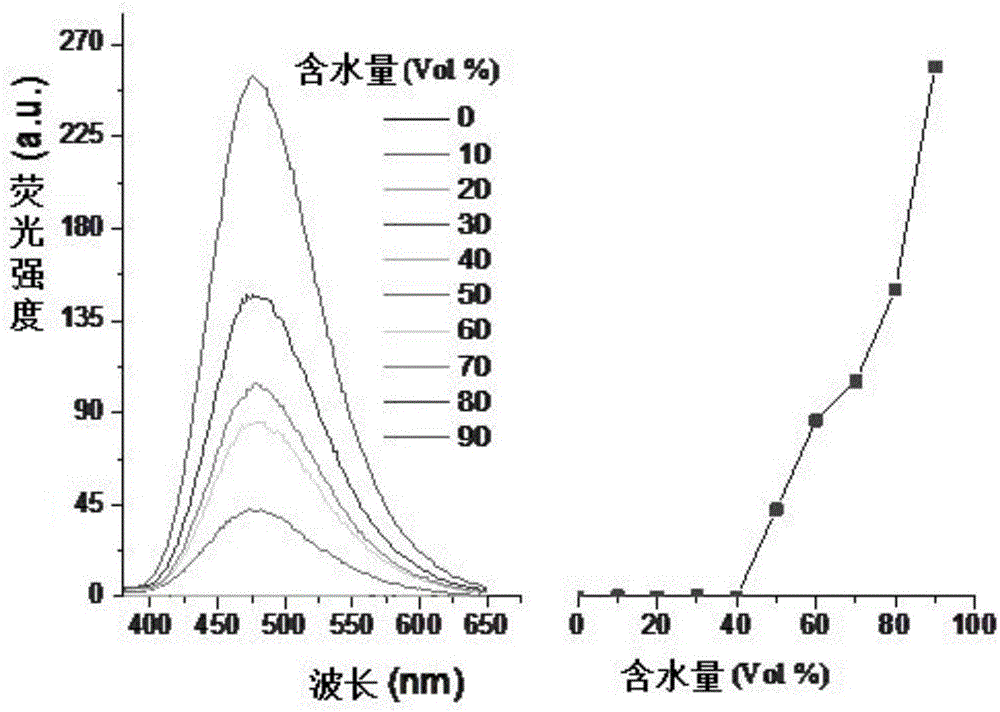
Image
Examples
Embodiment 1
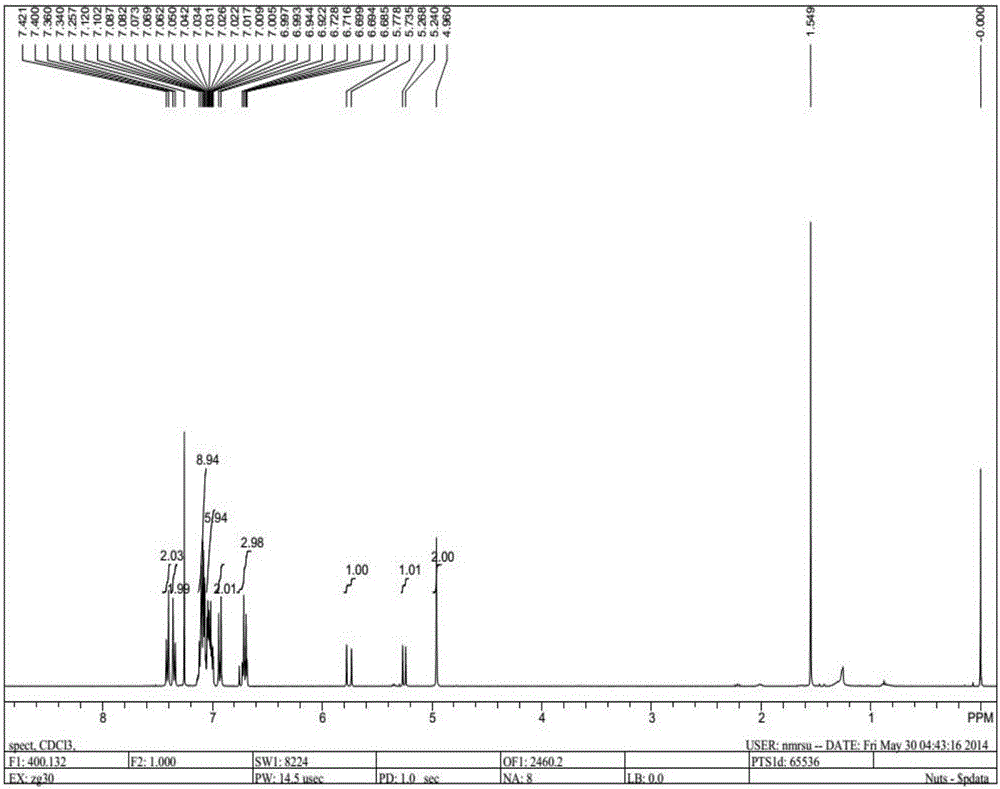
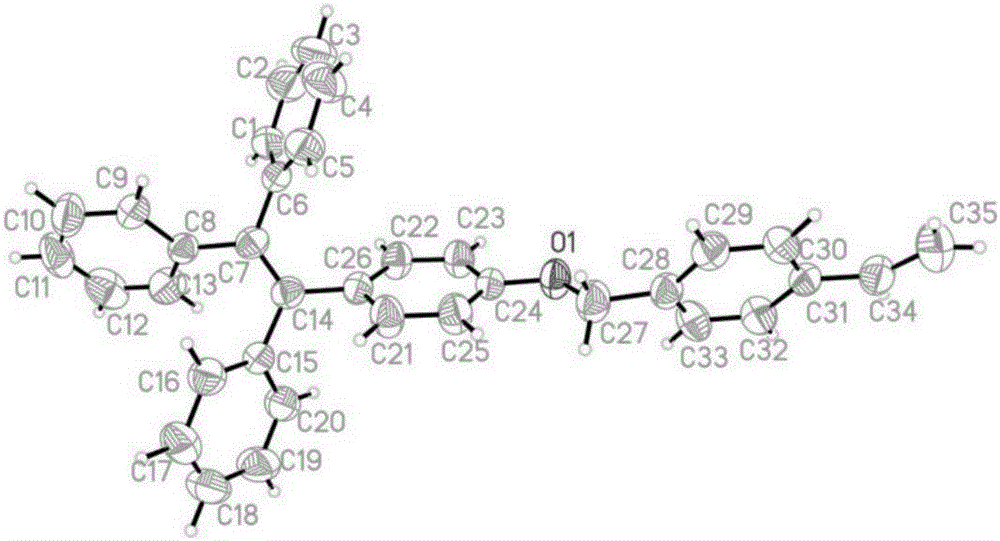
[0034] as attached figure 1 And attached figure 2 A fluorescent probe containing a tetraphenylethylene group as shown has a carbon-carbon double bond at the molecular end of the fluorescent probe, and the fluorescent probe comprises the following preparation steps:
[0035] (1) Under the conditions of nitrogen atmosphere and 0°C, 22 mmol of TiCl 4 Add dropwise to the tetrahydrofuran suspension solution containing 44 mmol of zinc powder, and stir at room temperature for 0.5 h; after refluxing for 2.5 h, cool down to 0°C to obtain a mixed solution;
[0036] (2) Dissolve 12mmol of benzophenone and 10mmol of 4'-hydroxyl-benzophenone in dry tetrahydrofuran, and add dropwise to the mixed solution prepared in step (1); reflux, TLC tracking reaction process;
[0037] (3) until the reaction in step (2) is completed, add water to quench the reaction, and the water layer is washed with CH 2 Cl 2 Extracted three times; the organic layer was taken and dried with anhydrous sodium sulf...
Embodiment 2
[0041] as attached figure 1 And attached figure 2 A fluorescent probe containing a tetraphenylethylene group as shown has a carbon-carbon double bond at the molecular end of the fluorescent probe, and the fluorescent probe comprises the following preparation steps:
[0042] (1) Under the conditions of nitrogen atmosphere and 0°C, 11 mmol of TiCl 4 Add dropwise to the tetrahydrofuran suspension solution containing 22mmol of zinc powder, and stir at room temperature for 0.5h; after refluxing for 2.5h, cool down to 0°C to obtain a mixed solution;
[0043] (2) Dissolve 6 mmol of benzophenone and 5 mmol of 4'-hydroxy-benzophenone in dry tetrahydrofuran, and add dropwise to the mixed solution prepared in step (1); reflux, TLC tracking reaction process;
[0044] (3) until the reaction in step (2) is completed, add water to quench the reaction, and the water layer is washed with CH 2 Cl 2 Extracted three times; the organic layer was taken and dried with anhydrous sodium sulfate,...
Embodiment 3
[0048] as attached figure 1 And attached figure 2 A fluorescent probe containing a tetraphenylethylene group as shown has a carbon-carbon double bond at the molecular end of the fluorescent probe, and the fluorescent probe comprises the following preparation steps:
[0049] (1) Under the conditions of nitrogen atmosphere and 0°C, 44 mmol of TiCl 4 Add dropwise to the tetrahydrofuran suspension solution containing 88 mmol of zinc powder, and stir at room temperature for 0.5 h; after refluxing for 2.5 h, cool down to 0°C to obtain a mixed solution;
[0050] (2) Dissolve 24mmol of benzophenone and 20mmol of 4'-hydroxyl-benzophenone in dry tetrahydrofuran, and add dropwise to the mixed solution prepared in step (1); reflux, TLC tracking reaction process;
[0051] (3) until the reaction in step (2) is completed, add water to quench the reaction, and the water layer is washed with CH 2 Cl 2 Extracted three times; the organic layer was taken and dried with anhydrous sodium sulf...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com