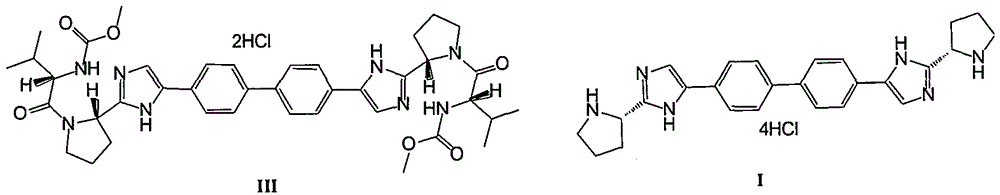
Preparation method of daclatasvir and its intermediate
A technology of daclatasvir and intermediates, which is applied in the field of preparation of daclatasvir and its intermediates, can solve the problems of harsh reaction conditions, high production cost, low product purity, etc., and achieves short reaction route and low production cost. , the effect of high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
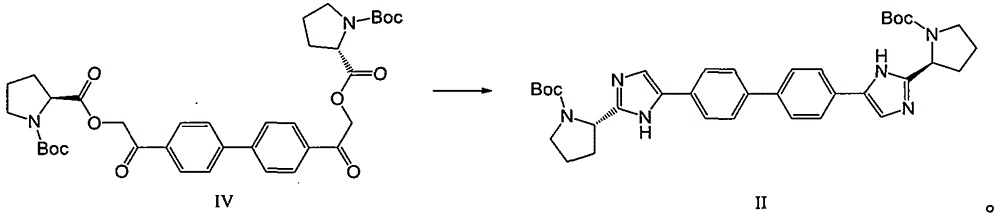
[0067] Embodiment 1: The preparation method of daclatasvir intermediate compound IV
[0068]
[0069] Add 5.00kg of 4,4'-bis(2-bromoacetyl)biphenyl, 5.98kg of BOC-L-proline and 32.00kg of acetonitrile into a 100L reactor equipped with mechanical stirring and a thermometer. Start stirring, control the reaction temperature to below 20°C, add 3.59kg of N,N-diisopropylethylamine dropwise, after the drop is complete, control the reaction temperature to 25°C to 30°C and stir for 3 hours. After completion of the reaction, it is 10% sodium chloride aqueous solution (described mass percent refers to the percentage of the quality of sodium chloride in the total mass of sodium chloride aqueous solution) that is pumped into the preconfigured mass percentage into 26.5kg, after stirring, leave standstill layered. The organic phase was repeatedly washed with water twice, and the layers were separated after standing. After the upper organic phase was separated, it was concentrated under ...
Embodiment 2
[0070] Embodiment 2: the preparation method of daclatasvir intermediate II
[0071]
[0072] Add 14.59 kg of ammonium acetate to a solution of 8.5 Kg of daclatasvir intermediate IV in 45.5 Kg (51 liters) of toluene, and raise the temperature to 95-100° C. After stirring for 15 hours, the temperature was lowered to 20-25°C, and 1.75Kg of glacial acetic acid, 8.14Kg of n-butanol and 20.1Kg of 13% sodium chloride aqueous solution were added by mass percentage (the mass percentage refers to that the mass percentage of sodium chloride accounted for The percentage of the total mass of sodium chloride aqueous solution) forms a mixed solution, which is stirred and left to stand for layering. Add 20.1Kg of 13% sodium chloride aqueous solution (the mass percentage refers to the percentage of the mass of sodium chloride in the total mass of sodium chloride aqueous solution) to the organic phase, stir and let stand to separate layers. The organic phase was decompressed and rotary evap...
Embodiment 3
[0073] Embodiment 3: the preparation method of daclatasvir intermediate I
[0074]
[0075] Add 5.00Kg daclatasvir intermediate II in 5 liters of methanol, 30 liters of dioxane, add dropwise 10L concentrated hydrochloric acid (mass concentration is 37%) under rapid stirring percentage of the total mass of hydrochloric acid), stirred at room temperature (25° C. to 30° C.) for 6 hours, and the reaction solution was filtered and washed. Add 20L methanol to the obtained wet product, heat up to 50-55°C and stir for 4 hours, cool to 15°C-20°C, stir for 1 hour, filter, wash, vacuum (-0.08MPa~-0.1 MPa) was dried for 6-10 hours to obtain 3.88Kg of daclatasvir intermediate I, with a yield of 85.0% and a HPLC purity of 99.68%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com