Method for preparing 2-deoxy-D-glucose
A technology of glucose and glucosamine hydrochloride, applied in the field of sugar chemistry, can solve the problems of complex process, low yield, high cost, etc., and achieve the effect of cheap and easy-to-obtain raw materials, fewer operation steps, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
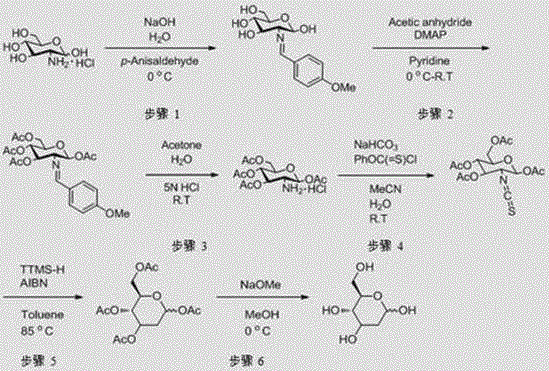
[0042] A preparation method of 2-deoxy-D-glucose, comprising the following steps:
[0043] ①Add glucosamine hydrochloride into water, stir to dissolve it, then add sodium hydroxide to it, stir evenly to obtain mixed solution 1, add p-anisaldehyde dropwise to mixed solution 1, and keep mixed solution 1 during the dropping process The temperature is 2~7°C, and the reaction is stirred at this temperature for 10~15 hours to obtain the reaction solution 1, and methyl tert-butyl ether is added to the reaction solution 1, stirred for 20~40 minutes, and suction filtered to obtain the filter cake 1 is 2-(4'-methoxybenzylidene)amino-2-deoxy-D-glucose, spare;
[0044] The mass volume ratio of the glucosamine hydrochloride, water, sodium hydroxide, p-anisaldehyde and methyl tert-butyl ether is 20~25g: 120~150ml: 4~4.5g: 10~15g: 180~200ml ;
[0045] ②Add 2-(4'-methoxybenzylidene)amino-2-deoxy-D-glucose obtained in step ① into pyridine to dissolve to obtain mixed solution 2, add acetic an...
Embodiment 1
[0084] A preparation method of 2-deoxy-D-glucose, comprising the following steps:
[0085] ① Add 40kg of glucosamine hydrochloride into 300L of water, stir to dissolve it, then add 8kg of sodium hydroxide to it, stir well to obtain mixed solution 1, add 20kg of p-anisaldehyde dropwise to mixed solution 1, during the dropwise addition process Keep the temperature of the mixed solution 1 at 2~7°C, and stir and react at this temperature for 10 hours to obtain the reaction solution 1, add 360L methyl tert-butyl ether to the reaction solution 1, stir for 20 minutes, and suction filter, the obtained solution is filtered Cake 1 is 2-(4'-methoxybenzylidene)amino-2-deoxy-D-glucose with a yield of 94%;
[0086] ②Take 20kg of 2-(4'-methoxybenzylidene)amino-2-deoxy-D-glucose obtained in step ① and add it to 65L pyridine to dissolve to obtain the mixed solution 2, and add 40kg of acetic anhydride to the mixed solution 2 dropwise , keep the temperature of the mixed solution 2 at 0~5°C duri...
Embodiment 2
[0092] A preparation method of 2-deoxy-D-glucose, comprising the following steps:
[0093] ① Add 100kg of glucosamine hydrochloride into 480L of water, stir to dissolve it, then add 18kg of sodium hydroxide to it, stir well to obtain mixed solution 1, add 60kg of p-anisaldehyde dropwise to mixed solution 1, during the dropping process Keep the temperature of the mixed solution 1 at 2~7°C, and stir the reaction at this temperature for 10~15 hours to obtain the reaction solution 1, add 800L methyl tert-butyl ether to the reaction solution 1, stir for 20~40 minutes, pump filter, and the resulting filter cake 1 is 2-(4'-methoxybenzylidene)amino-2-deoxy-D-glucose, with a yield of 95%;
[0094] ②Dissolve 50kg of 2-(4'-methoxybenzylidene)amino-2-deoxy-D-glucose obtained in step ① into 150L pyridine to obtain mixed solution 2, and add 100kg acetic anhydride to mixed solution 2 dropwise , keep the temperature of the mixed solution 2 at 0~5°C during the dropwise addition, then add 0.5k...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com