Recombinant vector for luciferase based on pCDH and application thereof
A technology of recombinant vector and luciferase, which is applied in the field of genetic engineering to achieve the effects of high infectivity, high sensitivity and low background noise
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: Construction of pCDH-CAR19 lentiviral vector
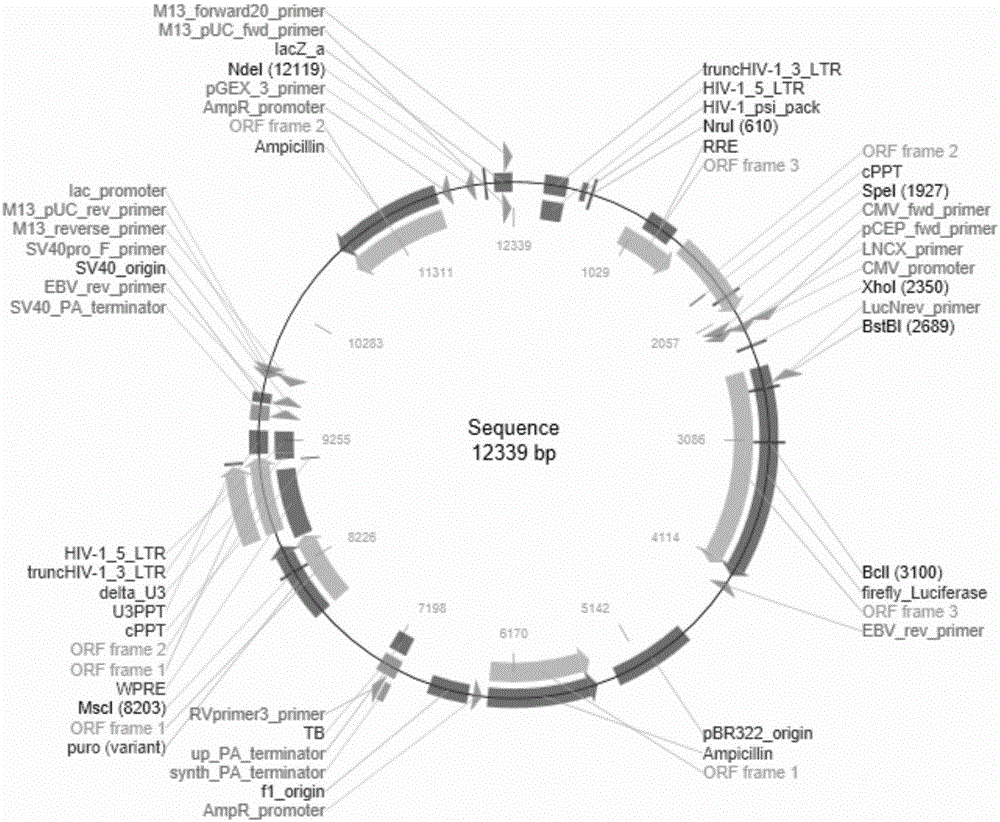
[0038] pCDH-Luc lentiviral vector, such as figure 1 Shown:
[0039] (1) According to the known pET28-Luc as the template to replace the primers at the restriction sites, the designed primers are as follows:
[0040] Upstream primer: CGCGGATCCATGGCCTTACCAGTGACCGCTCCGGT (BamHI-Luc-A);
[0041] Downstream primer: CCAGCGGCCGCAATCGCTCCCCCGTCCCGGA(Luc-NotI-S);
[0042] In order to facilitate the construction of the recombinant vector pCDH-Luc, we artificially added the enzyme cutting sites BamHI and NotI required for vector construction when synthesizing Luc.
[0043] The PCR amplification reaction system is as follows:
[0044]
[0045] Among them, a group of negative controls with water as the sample.
[0046] The reaction conditions are as follows:
[0047]
[0048]
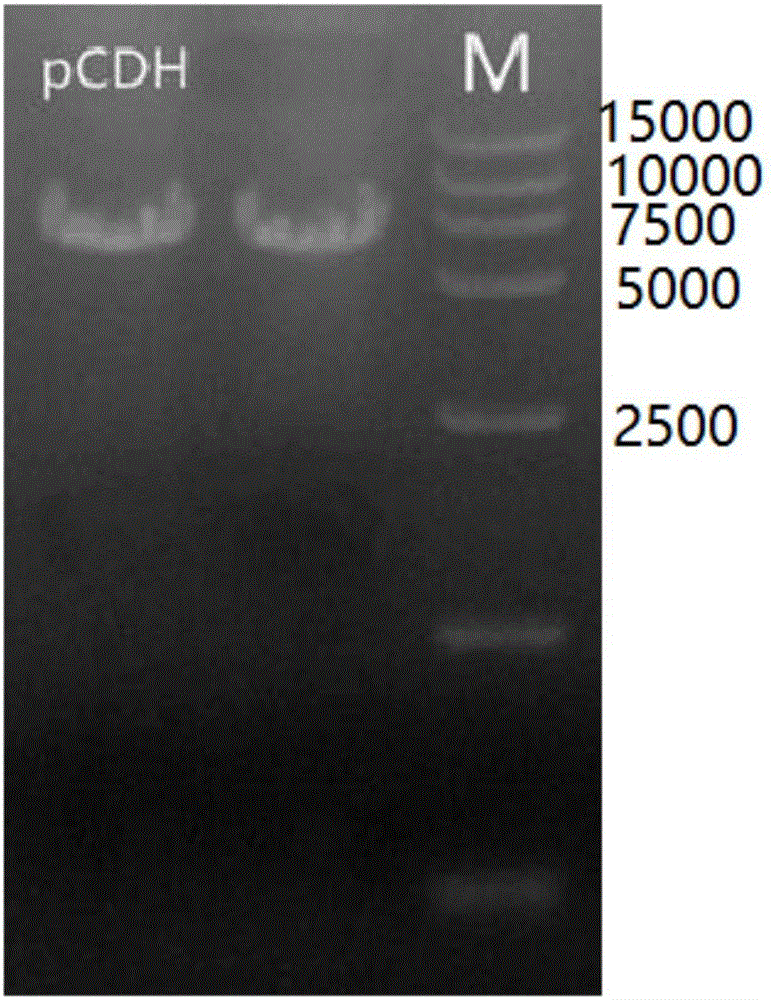
[0049] The obtained PCR product was identified by 1% agarose gel electrophoresis, recovered and purified with a gel recovery kit, NotI ...
Embodiment 2
[0056] Example 2: Mass extraction of pCDH-Luc plasmid
[0057] Take out the corresponding strains from -80°C, streak on the plate, and culture overnight (12-24h); pick a single clone (2-4) the next day, and shake the bacteria (6-8h) in a small volume (5ml); Take the bacterial liquid from the shaking system, and turn it to a large shake according to the volume ratio of 1:100; then carry out the plasmid extraction:
[0058] First confirm whether RNaseA has been added to Solution1 (save at 4°C after adding RNaseA); whether absolute ethanol has been added to DNAWashBuffer.
[0059] BufferN3 can be pre-cooled in ice before use; if Solution1 is not used for the first time, it needs to be taken out from 4°C and equilibrated at room temperature.
[0060] (1) Take an appropriate amount of bacterial solution and place it in a new 50mL centrifuge tube, centrifuge at 4900g for 10min at room temperature, discard the supernatant and continue to add an appropriate amount of bacterial soluti...
Embodiment 4
[0074] Example 4: Packaging of lentivirus and cell transfection
[0075] Lentivirus packaging: co-transfect 293T cells with the constructed lentivirus expression vector and packaging plasmid, package the virus, collect and concentrate the virus liquid, store and dilute, and perform titer detection and MOI value determination, titer > 1.00E+ 08TU / mL, MOI value between 5-10.
[0076] Cell transfection: (1) 24 hours before transfection, digest 293T cells in the logarithmic growth phase (cell confluency is 85%-95%) with trypsin (0.125%), and use complete medium (DMEM: 4.5g / Lglucose with NaHCO 3 or sodium pyruvate + 5% or 10% serum + 1mg / ml double antibody) to adjust the cell density (1:4-5) for passage, and re-seeded in culture vessels (T75 bottles or d15 culture dishes) for culture (37 ° C, 5% CO2, saturated humidity);
[0077] (2) After 24 hours of transfection, the cell confluence density reaches 45-65%;
[0078] (3) Replace the cell culture medium with fresh pure culture ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com