Application of specnuezhenide in preparation of medicine for treating neovascular diseases
A kind of technology of new blood vessel, special privet glycoside, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1 privetin to CoCl 2 Induced effects of VEGF overexpression
[0024] Experimental materials: DMEM / F12 medium (batch number 30-2006, Invitrogen Company); CoCl 2 (Lot No. 231-589-4, Sigma Company); 96-well plate (Lot No. 13313040, Corning Company); 20 × PBS solution (Lot No. 13112593Z, Shanghai Sangon Biological Company); Human VEGF Elisa kit (human vascular endothelial cell growth factor enzyme Linked immunoassay kit, batch number DVE00, R&D company); teruzhenin (batch number 111926-201102, specification: 20mg / bottle), provided by China Institute for the Identification of Pharmaceutical and Biological Products.
[0025] Take retinal pigment epithelial-19 cells (ARPE-19) in the logarithmic growth phase, and press 8×10 3 cells / well were inoculated in 96-well plate, and divided into 4 groups, namely, blank group, model group and teruzhenin group, with 3 multiple wells in each group; after the cells adhered to the wall, the old medium was discarded and replaced ...
Embodiment 2
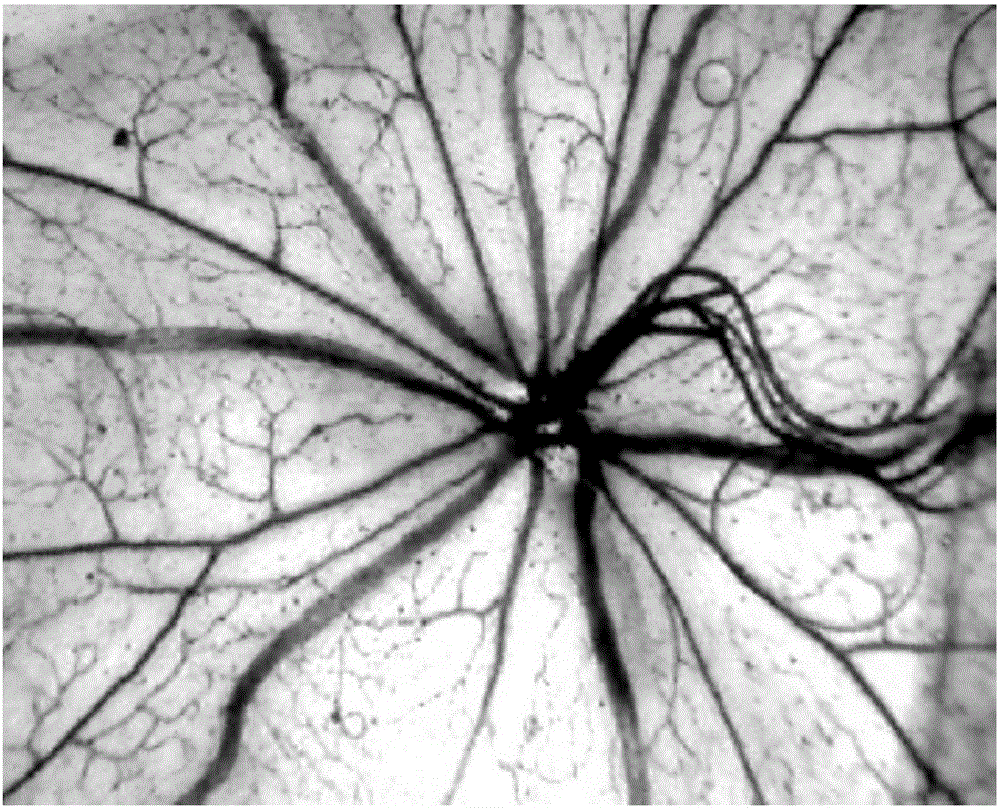
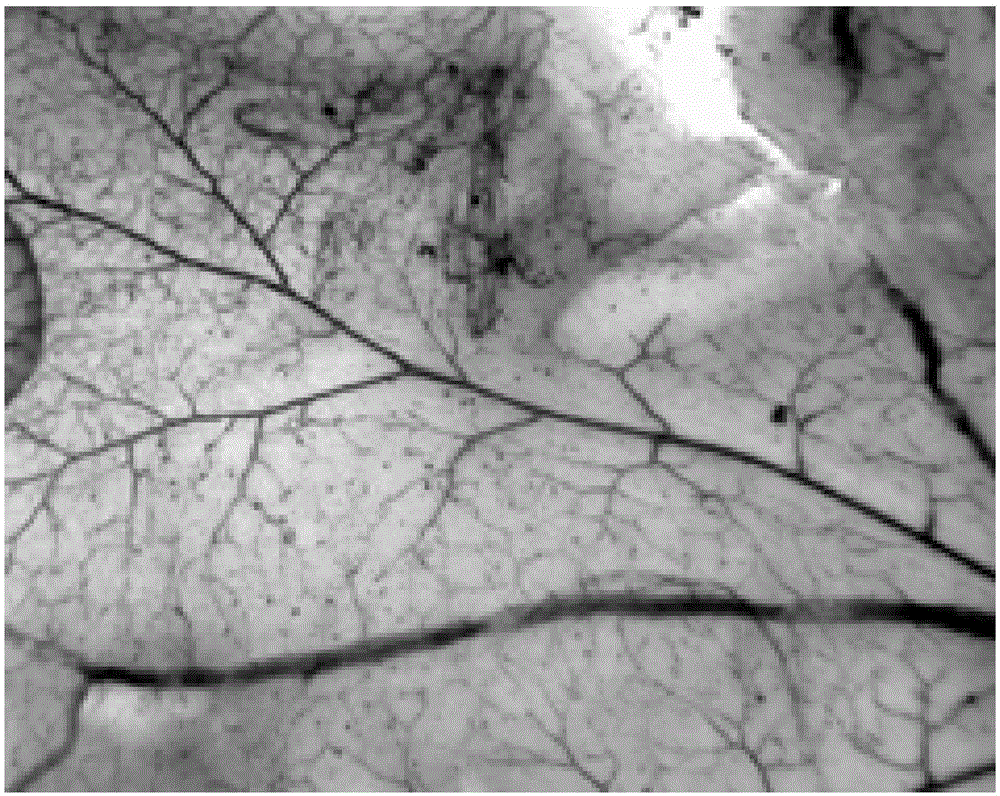
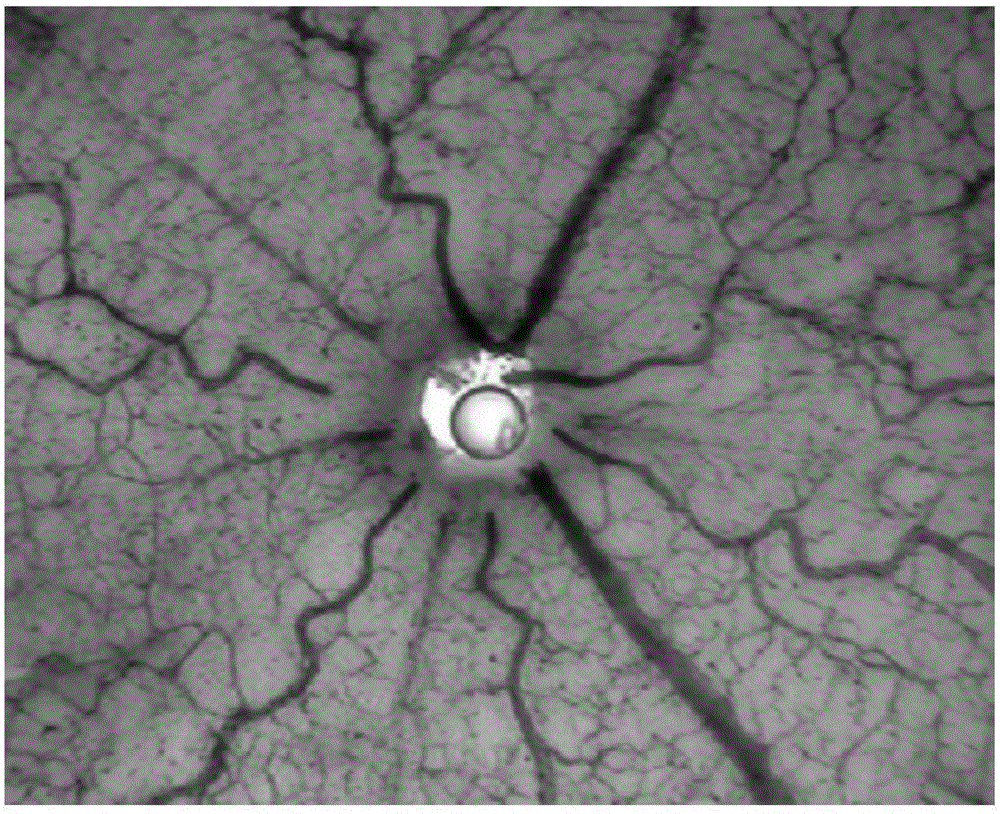
[0030] Example 2 Effects of terra privetin on hypoxia-induced retinal neovascularization
[0031] Experimental materials: Tris-maleic acid buffer (specification: 100mL, batch number JR02108, Shanghai Junrui Biotechnology Co., Ltd.); lead nitrate (specification: 500g, batch number 20130118, Chengdu Kelong Chemical Reagent Factory); magnesium chloride (specification: 500g, batch number 20130409, Chengdu Kelong Chemical Reagent Factory); glycerol buffer (specification: 500mL, batch number F20090908, Sinopharm Chemical Reagent Co., Ltd); Conbercept Ophthalmic Injection (specification: 10mg / ml, 0.2ml / Batch, batch number 20110610B, Chengdu Kanghong Biotechnology Co., Ltd.); privetin (batch number 111926-201102, specification: 20mg / bottle), provided by the National Institute of Pharmaceutical and Biological Products; SD pregnant rat, SPF grade, certificate number: SCXK (Sichuan) 2013-24, all provided by Chengdu Dashuo Biotechnology Co., Ltd.
[0032] Model establishment and group a...
Embodiment 3
[0040] Embodiment 3 terutrusin is to CoCl 2 Induced effects of HIF-1α overexpression
[0041] Experimental materials: YC-1 (specification: 10mg / cartridge, lot number ab120915, abcam company); DMEM / F12 medium (lot number 30-2006, Invitrogen company); CoCl 2 (Lot No. 231-589-4, Sigma Company); 96-well plate (Lot No. 13313040, Corning Company); 20 × PBS solution (Lot No. 13112593Z, Shanghai Sangon Biological Company); BCA kit (Lot No. MK165953, Thermo Scientific Company); Tinutrusin (batch number 111926-201102, specification: 20mg / bottle), provided by China National Institute of Pharmaceutical and Biological Products; protein lysate: (containing 50mmol / L Tris-HCl, 1% Triton X-100, 0.2% deoxycholesterol sodium phosphate, 0.2% SDS, 1mmol / L sodium edetate, 1mM sulfonyl fluoride, 1mmol / L PMSF, 10μg / ml leupeptin); 5× loading buffer (containing 250mM pH6.8Tris- HCl, 10% SDS, 0.5% bromophenol blue, 50% glycerol, 5% β-mercaptoethanol); tetramethylethylenediamine (TEMED; specification: ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com