Compound containing alkynyl, preparation method and application
A technology for compounds and carboxylate compounds, applied in the field of preparation of alkynyl esters of unsaturated carboxylic acids, can solve the problems of low activity of functional groups, increased separation and purification steps, low reaction conversion rate, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0101] Preparation of 1-ethynyl-(3-methyl)-cyclopentanol:
[0102] Add 130 grams of potassium tert-butoxide and 100 ml of cyclohexane into a 300 ml round bottom bottle at room temperature, pass through acetylene gas, add 100 grams of 3-methylcyclopentanone dropwise, stir and react at 15°C for 2 hours, add 20 ml of water, After stirring for 10 minutes, transfer to a 500ml separatory funnel, separate the water phase, then wash three times with distilled water, dry the organic phase with anhydrous sodium sulfate, and remove the solvent under reduced pressure. The yield is 92%.
preparation example 2
[0104] 3-Methyl-1-yne-3-pentanol, 3-ethynyl-3-decanol, 2-(p-butoxyphenyl)-3-yne-2-butanol, 2-phenyl-3 -Alkyne-2-butanol, 1-ethynyl-(4-methylthio)cyclohexanol, 2-(thiophene)-3-alkyne-2-butanol, 1-ethynylcyclohexanol, 1-ethynyl The preparation of base cyclopentanol:
[0105] Replace 3-methylcyclopentanone with the ketone corresponding to the prepared alcohol, and the molar ratio of the ketone to potassium tert-butoxide is 1:1.1; other conditions are the same as in Preparation Example 1.
Embodiment 1
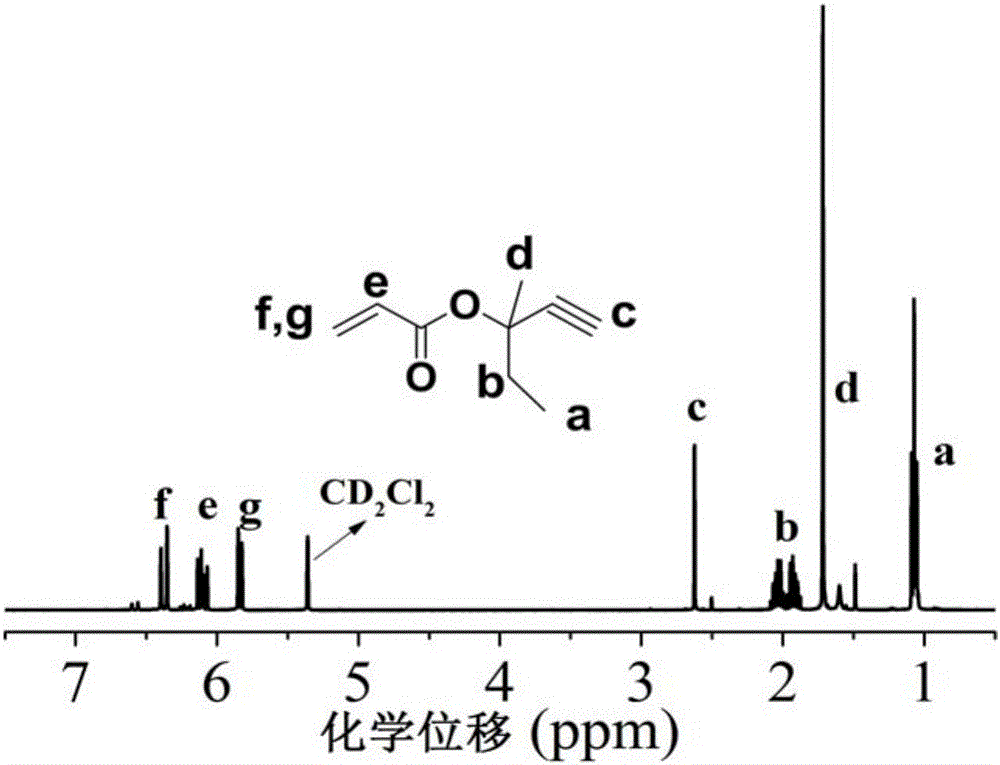
[0107] Acrylic acid (3-methyl-1-yne-3-pentyl) ester (R in the structural formula 1 =-CH 3 , R 2 =-C 2 h 5 , R 3 = R 4 =H, acid chloride esterification) preparation process is as follows:
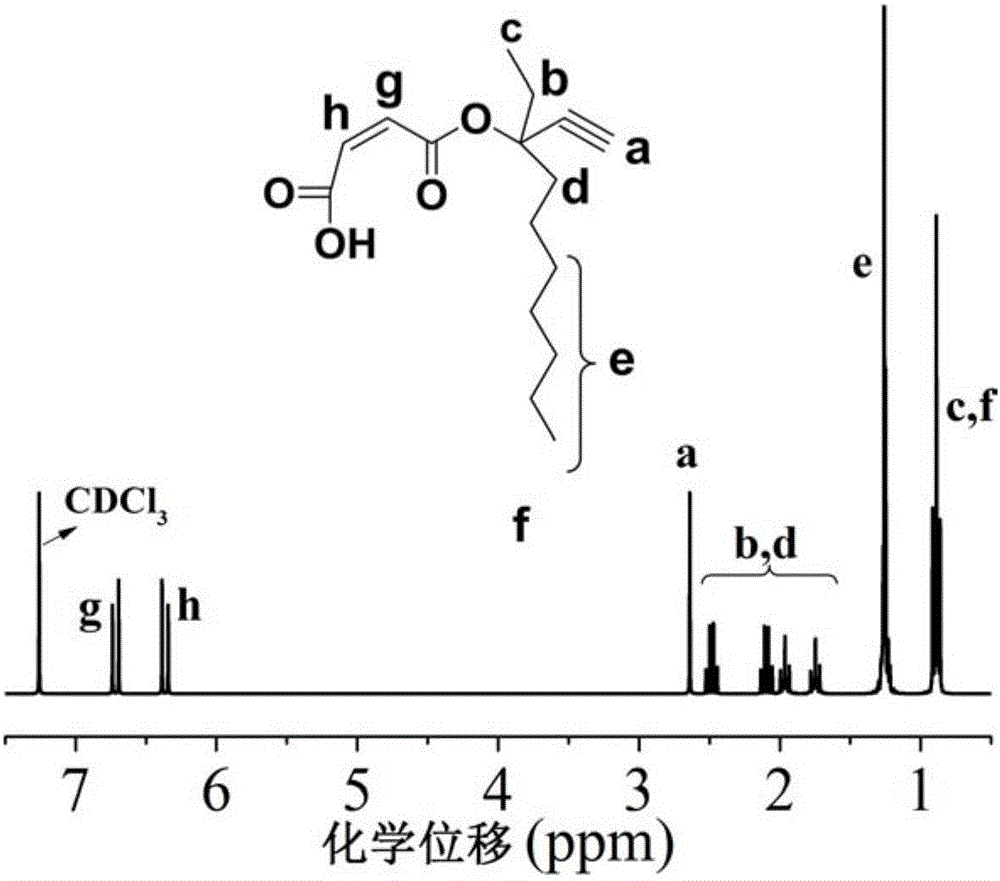
[0108] In a 500ml round bottom flask with magnetic stirring, add 35g (0.36mol) 3-methyl-1-yne-3-pentanol, 200ml dichloromethane and 34g (0.33mol) triethylamine, cool in an ice bath Add 26g (0.28mol) of acryloyl chloride dropwise, and react overnight at room temperature after the dropwise addition is completed for 2 to 3 hours. Suction filter the crude product, transfer the filtrate into a 1000ml separatory funnel, add 200ml of dichloromethane to dilute, and then use saturated Wash twice with sodium bicarbonate solution, then wash twice with deionized water, use a rotary evaporator to remove most of the solvent and distill under reduced pressure to obtain a colorless transparent liquid with a yield of 95%. Compound obtained in Example 1 1 H-NMR spectrum see figure 1 . The characteri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com