Calixarene-based hindered phenol antioxidant and preparation method and application thereof
A hindered phenolic and aromatic hydrocarbon-based technology, which is applied in ether preparation, organic chemistry, etc., can solve the problems affecting the anti-oxidation performance of polymer materials, affecting the anti-oxidation ability, and compatibility limitations, so as to improve the thermal-oxidative aging resistance. , Inhibit volatilization and migration, the effect that is not easy to play
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] (1) Synthesis of calixarene-based hindered phenolic antioxidants:
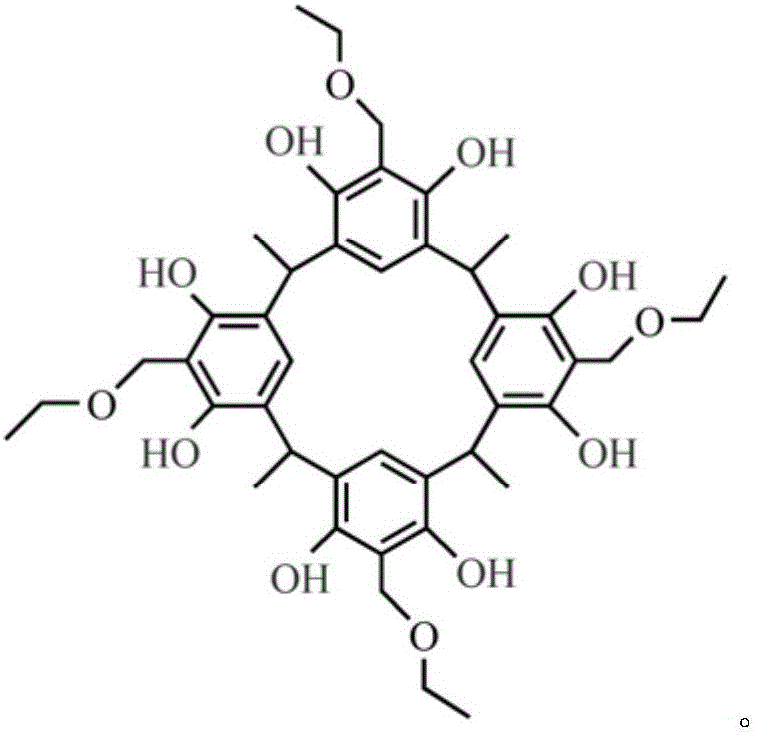
[0029] 0.5g C‐methyl resorcinol calix[4]arene (synthesized according to literature: Ito H, Nakayama T, SherwoodM, Miller D, Ueda M.Characterization and lithographic application of calix[4]resorcinarene derivatives.Chemistry of Materials, 2008,20(1):341‐356) and 0.5g of iminodiacetic acid were added to 12g of absolute ethanol, until the dissolution of C‐methylresorcinol calix[4]arene was completed, and 0.4g of mass concentration was added 37% formaldehyde solution. Then, under the condition of blowing nitrogen, the temperature was raised to 75° C. for 9 hours. After the reaction solution was cooled to room temperature, suction filtration was performed, and the filter residue was dissolved in 35 g of chloroform, and then insoluble iminodiacetic acid was removed by suction filtration to obtain a colorless filtrate. The filtrate was rotary evaporated, and vacuum-dried at 70° C. for 24 hours to obtain a ca...
Embodiment 2
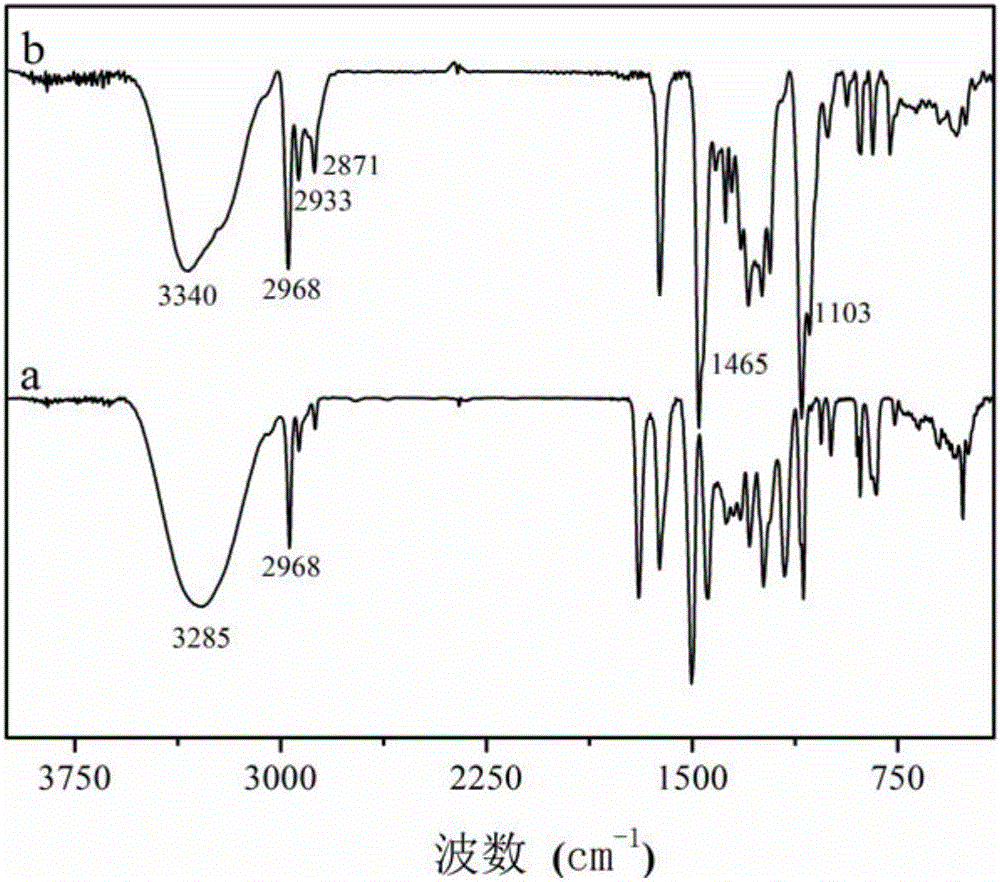
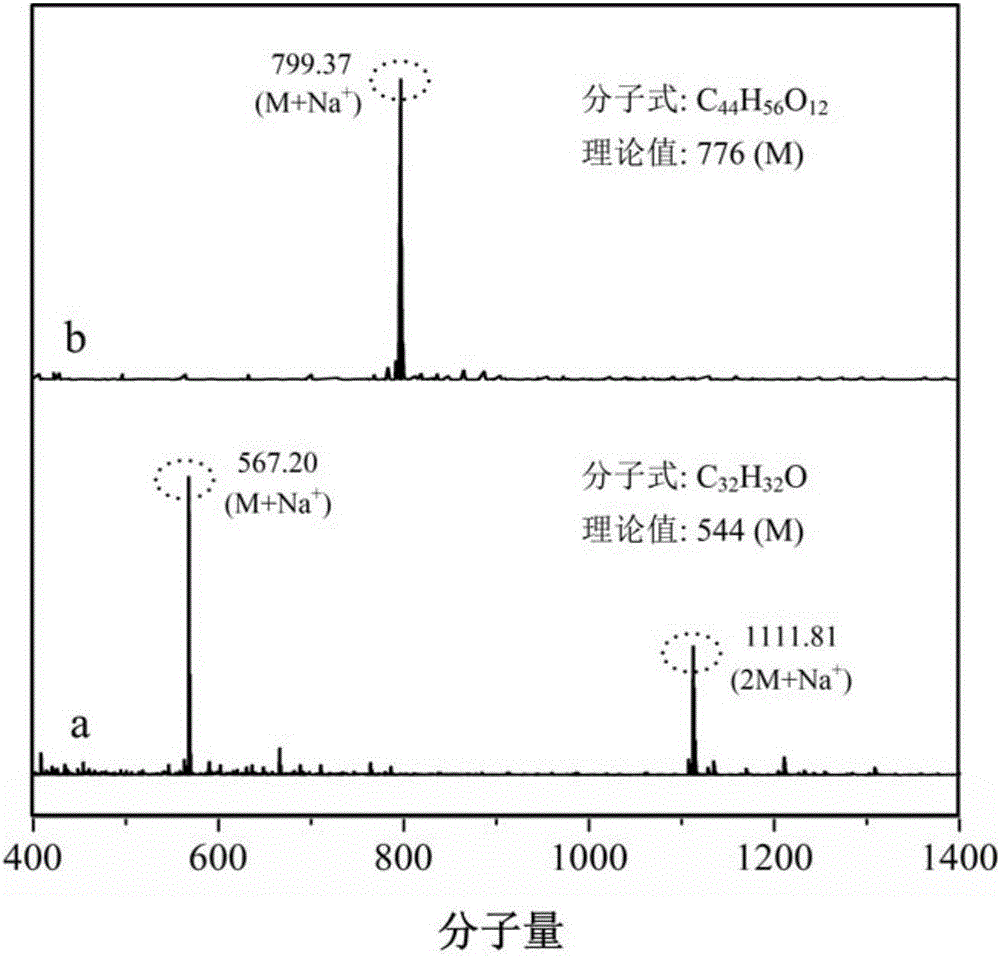
[0034] The difference between this embodiment and Example 1 is: the mass ratio of C‐methylresorcinol calix[4]arenes and absolute ethanol is changed to 1:15, that is, the consumption of absolute ethanol is changed to 7.5 g; the mass ratio of C‐methylresorcinol calix[4]arenes and 37% formaldehyde solution is changed to 1:0.5, that is, the quality of 37% formaldehyde solution is changed to 0.25g; C‐methylresorcinol The mass ratio of calix[4]arene and iminodiacetic acid is changed to 1:0.5, that is, the quality of iminodiacetic acid is changed to 0.25g; the quality of chloroform is changed to C‐methylresorcinol calix[4] 120 times the mass of the aromatic hydrocarbon, that is, changed to 60g; the reaction temperature was changed to 60°C, and the reaction time was changed to 24 hours. FT-IR and mass spectrometry are basically the same figure 1 , 2 , indicating the successful preparation of calixarene-based hindered phenolic antioxidants.
[0035] The thermo-oxidative aging resist...
Embodiment 3
[0037] The difference between this embodiment and Example 1 is: the mass ratio of C-methylresorcinol calix[4]arene and absolute ethanol is changed to 1:20, that is, the consumption of absolute ethanol is changed to 10g ; The mass ratio of C‐methylresorcinols calix [4]arene and 37% formaldehyde solution is changed to 1:3, that is, the quality of 37% formaldehyde solution is changed to 1.5g; C‐methylresorcinols The mass ratio of calix[4]arene to iminodiacetic acid was changed to 1:1, that is, the mass of iminodiacetic acid was changed to 0.5 g; the reaction temperature was changed to 78°C, and the reaction time was changed to 6 hours. FT-IR and mass spectrometry are basically the same figure 1 , 2 , indicating the successful preparation of calixarene-based hindered phenolic antioxidants.
[0038] The thermo-oxidative aging resistance and extraction resistance of natural rubber vulcanizates are shown in Table 1. It can be seen from Table 1 that the k value of natural rubber vu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com