A nido-carborane containing organometallic compound crystal and a preparing method thereof
An organometallic and carborane technology, which is applied in the field of nested carborane-containing organometallic compound crystals and the preparation thereof, can solve the problems of high temperature, poor repeatability, low yield and the like, and achieves low cost and mild reaction conditions. , the preparation method is simple
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
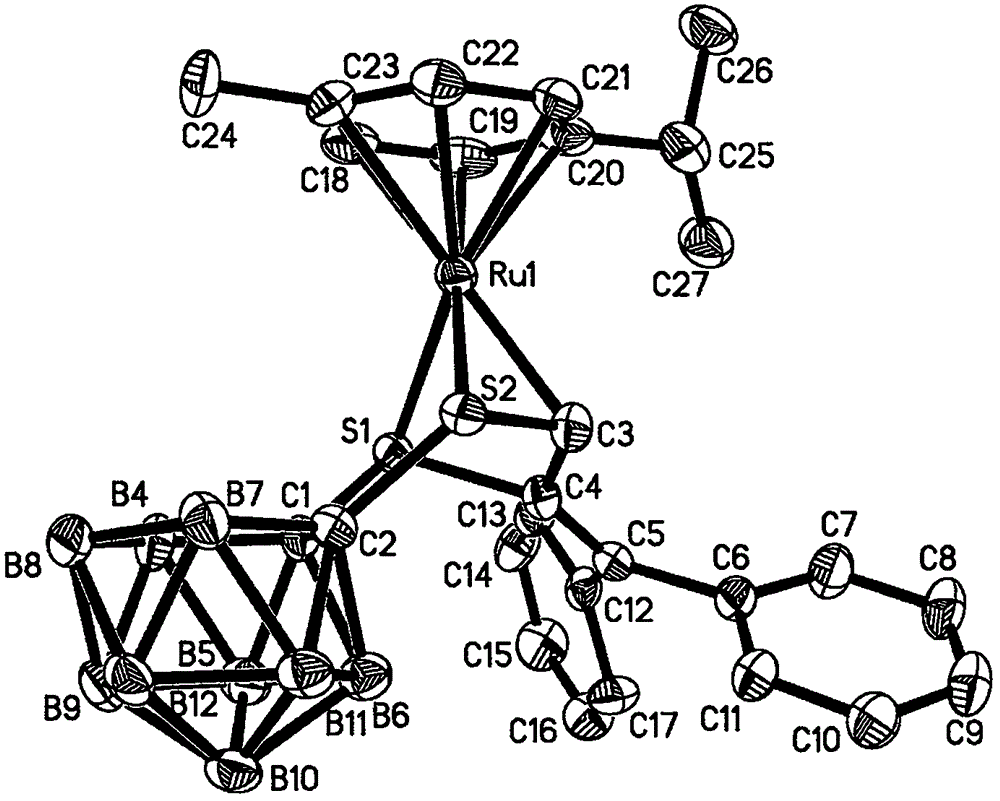
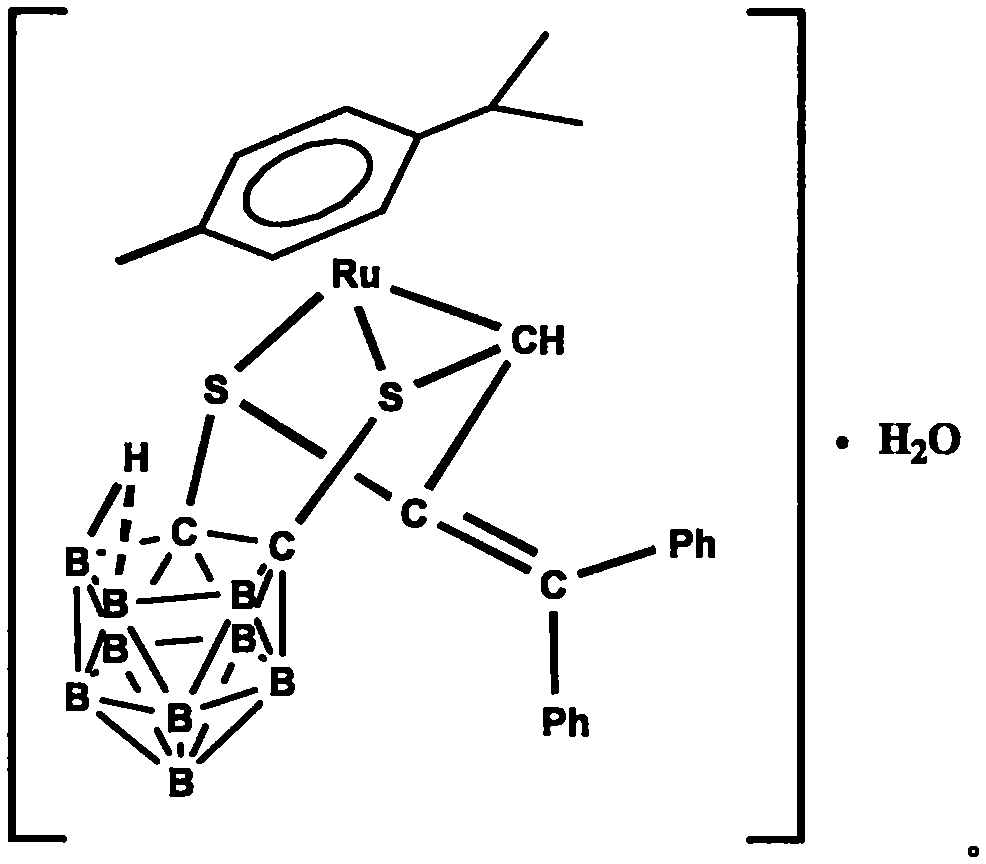
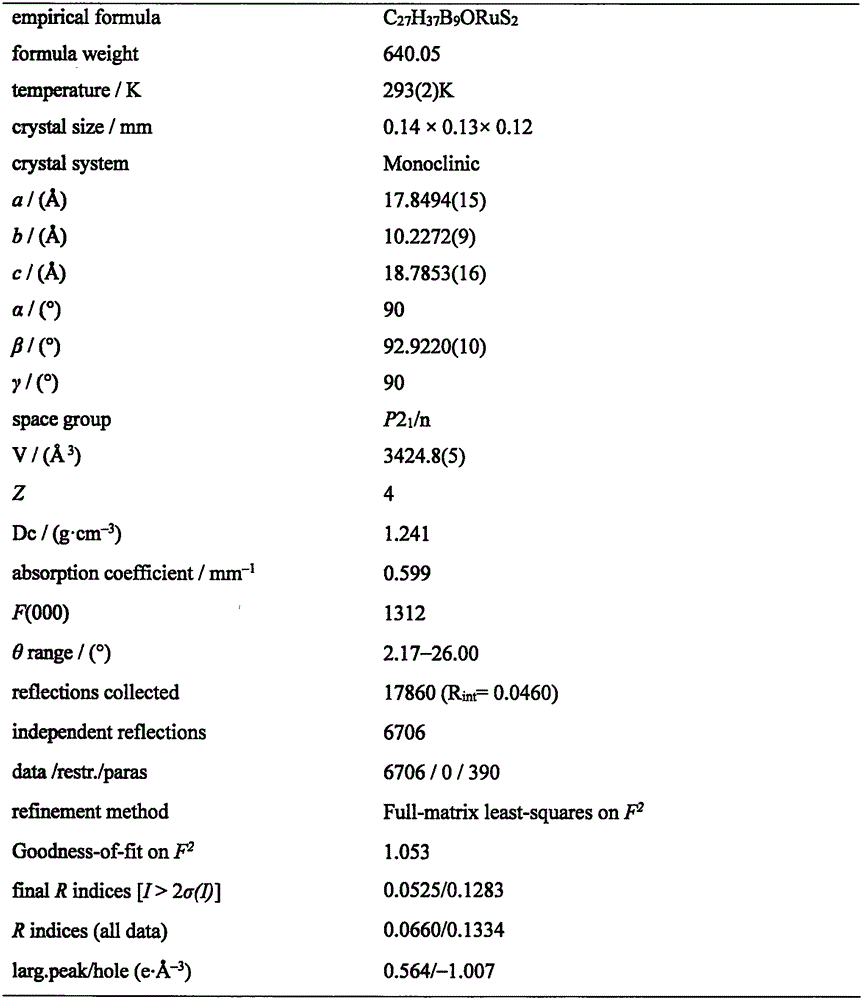
[0023] Under the protection of argon, 1,2-dicarbo-closed-dodecaborane (86 mg, 0.6 mmol) was dissolved in 20 mL of anhydrous ether, and n-butyllithium (2.0 mol·L -1 Cyclohexane solution) (0.6mL, 1.2mmol), sulfur powder (38.4mg, 1.2mmol), after stirring and dissolving, add dichloro(p-methylisopropylphenyl) ruthenium (II) dimer (185mg, 0.3mmol) tetrahydrofuran solution 40mL, ice-water bath temperature control 0 ℃, after 4 hours of reaction, the solvent was dried under vacuum; after dissolving the product with 20mL chloroform, add HC≡CC(OH)(Ph) 2(124.8mg, 0.6mmol), after 20 hours of reaction at 30°C with temperature control, the reaction solution was concentrated to dryness; the target compound (179mg, 48%) was obtained by separation on a 200-300 mesh silica gel column (eluent: V (petroleum ether) ) / V (methylene chloride)=2:1); the compound was dissolved in a mixed solvent of n-hexane and methylene chloride for crystallization (V (hexane) / V (methylene chloride)=1:1), Obtain yello...
Embodiment 2
[0025] Under the protection of argon, 1,2-dicarbo-closed-dodecaborane (86 mg, 0.6 mmol) was dissolved in 20 mL of anhydrous ether, and n-butyllithium (2.0 mol·L -1 Cyclohexane solution) (0.6mL, 1.2mmol), sulfur powder (38.4mg, 1.2mmol), after stirring and dissolving, add dichloro(p-methylisopropylphenyl) ruthenium (II) dimer (185mg, 0.3mmol) tetrahydrofuran solution 40mL, ice-water bath temperature control 0 ℃, after 4 hours of reaction, the solvent was vacuum-dried; after dissolving the product with 20mL chloroform, add HC≡CC(OH)(Ph) 2 (124.8mg, 0.6mmol), after 18 hours of reaction at 35°C with temperature control, the reaction solution was concentrated to dryness; the target compound (183mg, 49%) was obtained by separation on a 200-300 mesh silica gel column (eluent: V (petroleum ether) ) / V (methylene chloride)=2:1); the compound was dissolved in a mixed solvent of n-hexane and methylene chloride for crystallization (V (hexane) / V (methylene chloride)=1:1), Obtain yellow blo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com