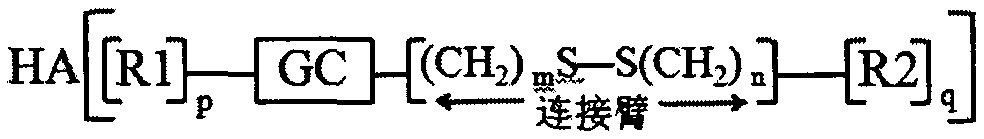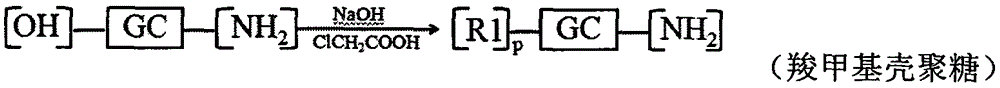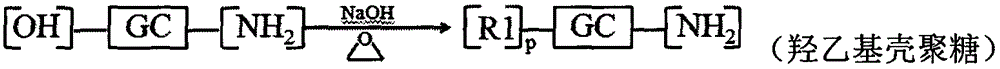Preparation and application of hyaluronic acid-modified amphipathic chitosan derivative carrier with tumor microenvironment specificity drug release effect
A chitosan derivative, hyaluronic acid modification technology, applied in the direction of antitumor drugs, drug combination, drug delivery, etc., to achieve the effects of high drug loading, increased stability and biocompatibility, and high encapsulation efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0085] Embodiment 1: Preparation of hyaluronic acid modified hydroxyethyl chitosan-n-octylamine reduction sensitive copolymer
[0086] Dissolve 1.000g chitosan in 2% acetic acid aqueous solution, add 50% NaOH solution in batches, alkalinize at 40°C for 12h, add appropriate amount of ethylene oxide in batches under ice bath conditions, react at 0°C for 2-4h, Raise the temperature to 35-50° C. to continue the reaction for 24 hours, adjust the pH value to 6-8 with concentrated hydrochloric acid, dialyze (MWCO=14000) for 48-72 hours, and freeze-dry to obtain hydroxyethyl chitosan.
[0087] Dissolve 0.300g hydroxyethyl chitosan in a mixed solution of water and methanol (v / v=1:1), dissolve 0.600g 3,3'-dithiodipropionic acid in methanol solution, add 0.547 g 1-ethyl-(3-dimethylaminopropyl) carbodiimide (EDC) and 0.328g hydroxysuccinimide (NHS), after activation for 0.5-1h, slowly drop into aqueous solution of hydroxyethyl chitosan , react at room temperature for 24 hours, remove met...
Embodiment 2
[0090] Embodiment 2: Preparation of carboxymethyl chitosan-n-octanoic acid reduction-sensitive copolymer modified by hyaluronic acid
[0091] Dissolve 1.000g chitosan in isopropanol solution, add 50% NaOH solution in batches, alkalinize at 40°C for 12h, add 6.000g chloroacetic acid, react at 40°C for 24h, pour off the supernatant, add appropriate amount of water, Make it a clear and transparent yellow solution. Concentrated hydrochloric acid was used to adjust the pH value to 6-8, dispersed in methanol, suction filtered, the filter cake was redissolved in water, dialyzed (MWCO=14000) for 48-72 hours, and freeze-dried to obtain carboxymethyl chitosan.
[0092] Dissolve 0.300g carboxymethyl chitosan in water, dissolve 0.500g 3-[(2-aminoethyl)dithio]propionic acid in methanol solution, add 0.547g 1-ethyl-(3-di Methylaminopropyl) carbodiimide (EDC) and 0.328g hydroxysuccinimide (NHS), after activation for 0.5-1h, slowly drop into hydroxyethyl chitosan aqueous solution, react at r...
Embodiment 3
[0095] Embodiment 3: Preparation of hydroxyethyl chitosan-cholic acid reduction-sensitive copolymer modified by hyaluronic acid
[0096] Dissolve 1.000g of chitosan in 2% acetic acid aqueous solution, add an appropriate amount of 50% NaOH solution, alkalinize at 40°C for 12 hours, add appropriate amount of ethylene oxide in batches under ice bath conditions, react at 0°C for 2-4 hours, and heat up Continue the reaction at 35-50°C for 24 hours, adjust the pH value to 6-8 with concentrated hydrochloric acid, dialyze (MWCO=14000) for 48-72 hours, and freeze-dry to obtain hydroxyethyl chitosan.
[0097] Dissolve 0.300g of hydroxyethyl chitosan in a mixed solution of water and methanol (v / v=1:1), and dissolve 0.500g of 3-[(2-aminoethyl)dithio]propionic acid in methanol Add 0.547g of 1-ethyl-(3-dimethylaminopropyl) carbodiimide (EDC) and 0.328g of hydroxysuccinimide (NHS) into the solution, activate it for 0.5-1h, then slowly drop in hydroxyethyl Chitosan-based aqueous solution, re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com