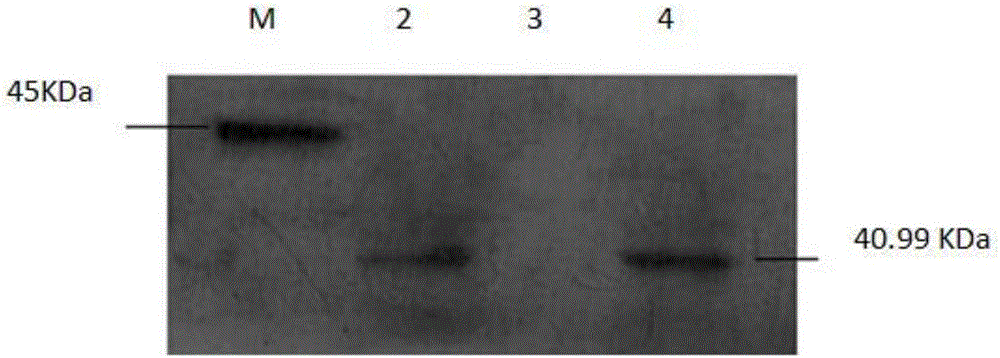
Recombinant staphylococcus aureus ClfA protein vaccine and construction method of eukaryotic expression engineering cell line of protein vaccine
A staphylococcus and protein vaccine technology, applied in other methods of inserting foreign genetic materials, recombinant DNA technology, genetic engineering, etc., can solve the problems of difficult expression and purification of protein products, and achieve single antigenic characteristics, strong feasibility, The effect of design science
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1. The construction method of eukaryotic expression engineering cell line is as follows:
[0034] Step 1. According to the genome sequence of Staphylococcus aureus published online by NCBI, design ClfA gene primers; wherein, the upstream primer ClfA-1168F1, the sequence is GACA AAGCTT AATATGGGCGAAACGAGTG, the downstream primer ClfA-1168R1, the sequence is CTCGAG ATCAATTTGGATTTGGGAA.
[0035] Step 2, taking the genomic DNA of Staphylococcus aureus (coded as MD2) as a template, extracting the genomic DNA, and amplifying the ClfA gene sequence with primers ClfA-1168F1 and ClfA-1168R1.
[0036] The extraction method for the genomic DNA of Staphylococcus aureus is:
[0037] 1. Cultivate 3ml of bacterial culture overnight, collect the bacterial cells by centrifugation at 13000g at room temperature for 1min, resuspend the bacterial cells with 160μl Buffer EB, then add 40μl lysozyme (100mg / ml), vortex and mix, place at 37°C for 30min; centrifuge at 5000g 3min and...
Embodiment 2
[0155] The difference between this example and Example 1 is that the double enzyme digestion system of the ClfA gene and the carrier pcDNA 3.1 V5-His B in step 3 is 50 μl, and the enzyme digestion reaction condition is overnight enzyme digestion at 2-8°C.
[0156] The double enzyme digestion reaction system involved is:
[0157]
[0158] Pipette well, then add restriction endonuclease
[0159] Hind III (10u / μl) 0.5μl
[0160] Xho I (10u / μl) 0.7μl
[0161] Pipette evenly with a 10μl pipette, cover the cap of the centrifuge tube, and centrifuge briefly to fully mix the components; digest overnight at 2-8°C.
[0162] The ligation reaction system of ClfA and pcDNA 3.1 V5-His B after enzyme digestion is 20 μl, and the reaction conditions are 16°C overnight ligation reaction system and reaction conditions (1.5mL centrifuge tube):
[0163]
[0164] Connect overnight at 16°C for 8-16 hours, generally you can get better results.
[0165] In step 6, when using Ni-NTA affinity ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com