Fluorine-containing water-soluble platinum complex and its preparation method and use
A platinum complex and water-soluble technology, which is applied in the field of fluorine-containing water-soluble platinum complexes and its preparation, can solve the problems of tumor targeting and anti-tumor effects to be improved, and achieve poor stability of preparations, improve targeting, The effect of high water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
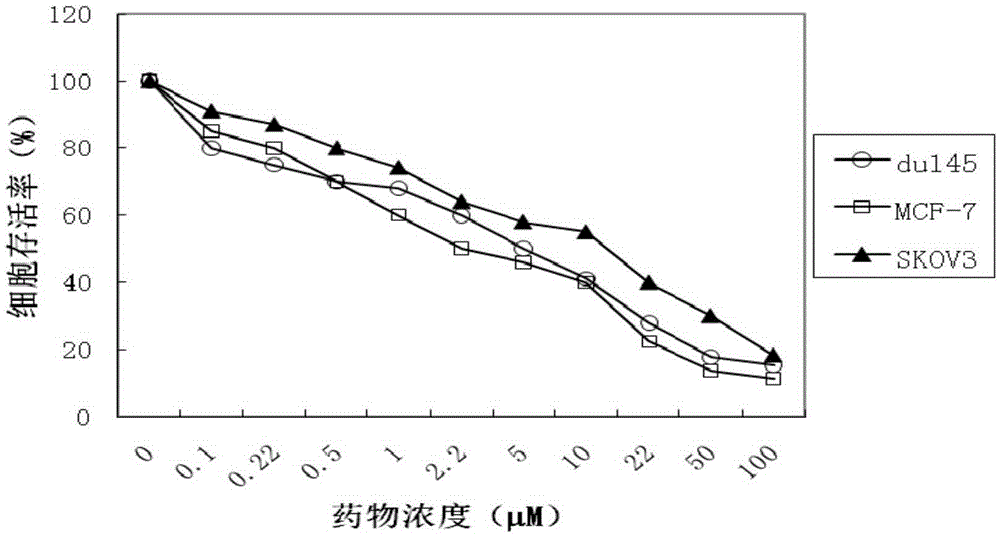
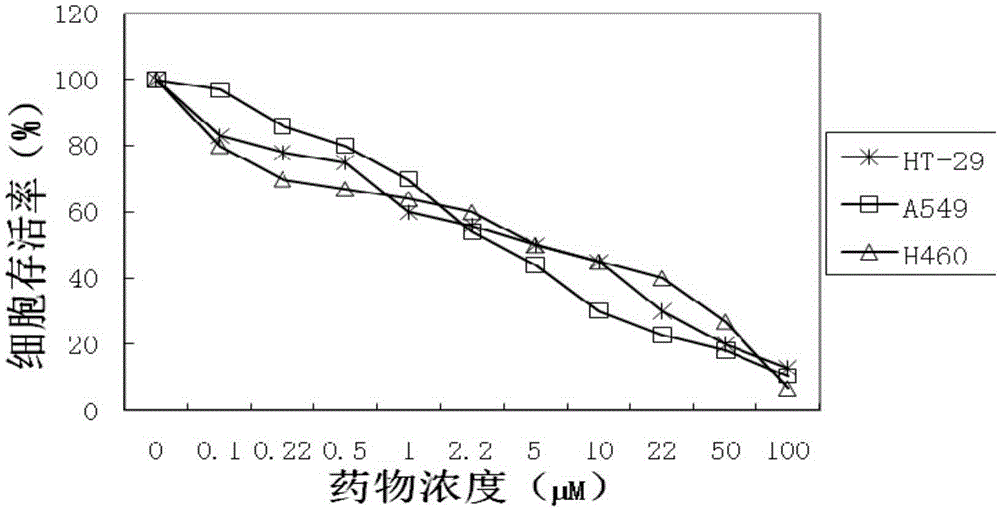
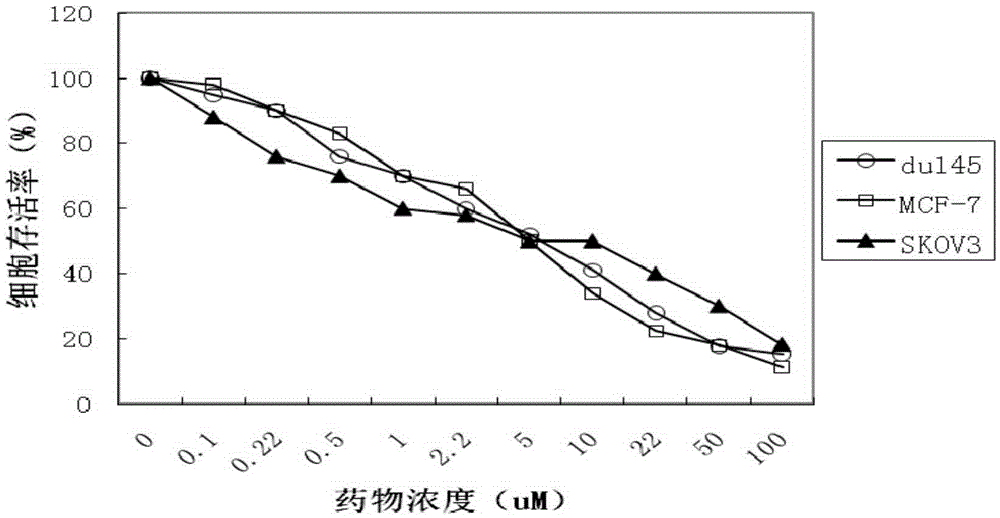
Examples
preparation example Construction
[0083] The preparation method shown in method C is to condense halohydrin with acetylated deoxyglucose in the presence of Lewis acid, then carry out substitution reaction with malonate derivatives, and finally obtain compound (III) after deprotection reaction route. The acetylation of deoxyglucose involved in the above preparation route can be carried out according to the methods reported in the literature, for example, using acetic anhydride in pyridine as the acetylation reagent and heating at room temperature or 60° C. for 1-24 hours can be completed. The condition of the glycoside synthesis reaction in the presence of Lewis acid is to use 0.1-50 equivalents of fluorine-containing malonate derivatives for deoxyglucose compounds, or conversely use 0.1-50 equivalents of deoxyglucose raw materials for fluorine-containing malonate derivatives. The Lewis acid used can be BF 3 , SnCl 4 , FeCl3 , AlCl 3 , hydrochloric acid, p-toluenesulfonic acid, camphorsulfonic acid, etc., th...
Embodiment 1
[0087] (1) Preparation of 1-O-(3,4,6-triacetyl-D-2-deoxyglucoside)-2-bromo-ethane (V-1):
[0088]
[0089] At room temperature, 1.6 g of D-2-deoxyglucose was dissolved in pyridine and acetic anhydride (7 ml: 7 ml), stirred overnight, and the end point of the reaction was monitored by TLC. After the reaction was completed, 100 ml of ethyl acetate was added, washed with 5% aqueous hydrochloric acid (2×25 ml), the aqueous phase was extracted with ethyl acetate (2×25 ml), and the organic phases were combined. The organic phase was washed successively with saturated aqueous ammonium chloride solution (1×100ml), distilled water (1×100ml), saturated aqueous sodium bicarbonate solution (1×100ml), saturated aqueous sodium chloride solution (1×100ml), and washed with anhydrous sulfuric acid Sodium dry. The solvent was evaporated to dryness with a rotary evaporator to obtain a slightly yellow crude product of 3,4,6-triacetyl-D-2-deoxyglucose. Dissolve the obtained crude product in 2...
Embodiment 2
[0111] Preparation of diaminoplatinum (II) [1-O-(D-2-deoxyglucoside)-propane-3-fluoro-3,3-dicarboxylate] (I-2):
[0112]
[0113] 1) Dissolve 110 mg of crude product 1-O-(D-2-deoxyglucoside)-propane-3-fluoro-3,3-dicarboxylic acid (III-1) in 5 ml of deionized water, add hydrogen octahydrate Barium oxide (about 98 mg dissolved in 5 ml of water) was used to adjust the pH of the reaction solution to 8, and stirred at room temperature for 30 minutes.
[0114] 2) Dissolve platinum diaminosulfate (115 mg) in 2 ml of water under nitrogen protection, add to the reaction solution in 1), adjust the pH to 8 with barium hydroxide solution, and stir at room temperature overnight in the dark.
[0115] 3) After the reaction is completed, use a centrifuge to remove the precipitate, collect the supernatant, use semi-preparative HPLC to refine and separate, and use a freeze dryer to freeze-dry to obtain 100 mg of the final product as a white solid.
[0116] NMR spectrum (400MHz, D2O), ppm: 4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com