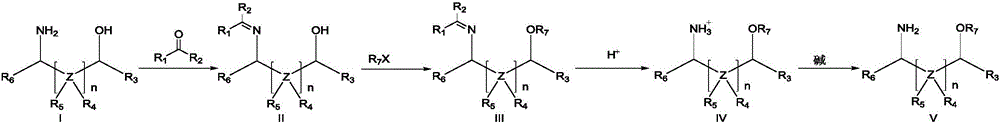
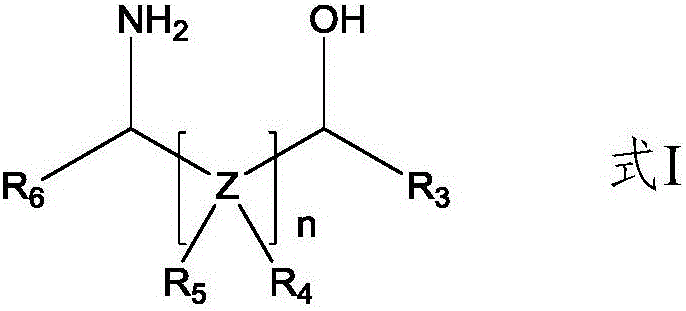
Method for preparing amino ether compounds
A technology for amino ethers and compounds, which is applied in the field of preparation of amino ether compounds, can solve the problems of high price, cumbersome post-processing, poor selectivity and the like, and achieves the effects of low production cost, wide application range and good yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Embodiment 1: the preparation of 3-amino-1-propyl methyl ether
[0057] (1) Add benzaldehyde (1.00mol, 106g) to 300g cyclohexane solvent, add 3-amino-1-propanol (1.1mol, 82.50g), reflux at 90°C, add water divider to separate water, and react 3h, after the reaction was completed, the cyclohexane was distilled off to obtain 162.0 g of white crystalline solid A1 with a yield of 99.38% and a purity of 99.6%.
[0058] (2) 162.0gA1 is added in 500g toluene solvent, add 18-crown-6 phase transfer catalyst (6mmol, 1.62g, 0.6%), KOH (1.5mol, 84g), control reaction system temperature at -5~10 Between ℃, iodomethane (1.1mol, 157g) was added dropwise, the dropwise addition was completed in 2.5h, and the reaction was complete in 3.5h. Add 100 g of water, extract and separate the layers, wash the toluene layer once with 50 g of water, and then distill the toluene layer off to obtain 174 g of white solid B1; the yield is 99.43%, and the purity is 99.15%.
[0059] (3) Add 174g of B1 t...
Embodiment 2
[0061] Embodiment 2: the preparation of D-valline ether
[0062] (1) Add benzaldehyde (3.00mol, 318g) to 1000g toluene solvent, add D-valinol (3.3mol, 340.46g, ee value, 99.5%) to reflux, add water divider to remove water, and reflux for 4h After the reaction was completed, toluene was distilled off for post-treatment to obtain 568.30 g of white crystal A2 with a yield of 99.8% and a purity of 99.10%.
[0063] (2) 568.30gA2 is joined in 5000g tetrahydrofuran solvent, add 15-crown ether-5 phase-transfer catalyst (18mmol, 4g, 6%), NaOH (4.00mol, 160g) powder, control reaction system temperature at-5~10 Between ℃, (3.3mol, 416g) dimethyl sulfate was added dropwise, and the dropwise addition was completed within 4 hours. The cooling was removed to allow the reaction temperature to gradually rise to room temperature, and the reaction was carried out for 5 hours. After the reaction was completed, the solvent was removed, and the organic layer was washed with water to obtain 605.25...
Embodiment 3
[0066] Embodiment 3: L-amphetamine ether
[0067] (1) Benzaldehyde (1.10mol, 116g) was added to 500g xylene solvent, L-phenylalaninol (1.0mol, 151g, ee value, 99.8%) was added, and the water removal device was placed on top, and the reaction was refluxed for 4h, After the reaction was completed, xylene was distilled off under reduced pressure to obtain 237.00 g of white crystal A3 with a yield of 99.08% and a purity of 99.58%.
[0068] (2) 237gA3 is joined in 2400g n-hexane solvent, add TBAB (1mmol, 0.37g, 0.1%) phase transfer catalyst, NaOH (2.48mol, 99.08g), control reaction system temperature between-5~5 ℃, Benzyl chloride (1.19mol, 149.96g) was added dropwise, and the addition was completed within 1.5h. After 0.5 hours of reaction, the cooling was removed to allow the reaction temperature to gradually rise to room temperature, and the reaction was carried out for 3.5 hours. After the reaction was completed, 530 g of water was added, extracted and separated, and n-hexane ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com