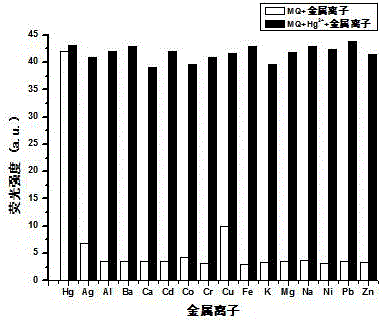
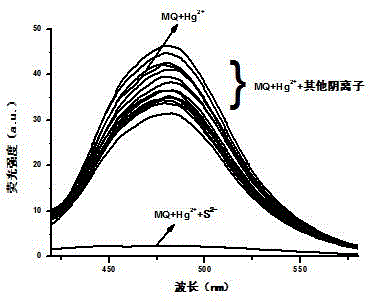
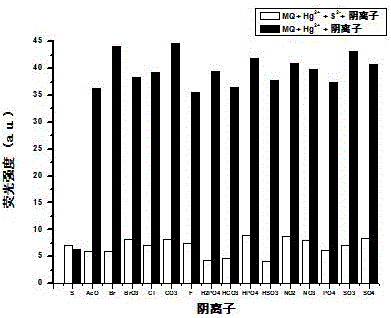
Reversible di-hydroxyl phenanthroimidazole Hg<2+> fluorescence probe, and synthesis and use methods
A fluorescent probe, bishydroxyphenanthrene technology, applied in fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problems of lack of reversible recognition performance, recognition interference, etc., and achieve the effect of high selectivity and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0026] Specific embodiment one: the reversible bishydroxyphenanthroimidazole Hg of this embodiment 2+ The structural formula of the fluorescent probe is:
[0027]
specific Embodiment approach 2
[0028] Specific embodiment two: the reversible bishydroxyphenanthroimidazole Hg of specific embodiment one 2+ The preparation method of fluorescent probe is carried out according to the following steps:
[0029] 1. Intermediate compound Synthesis:
[0030] Add phenanthrenequinone, 5-nitrosalicylaldehyde and ammonium acetate into the reactor at a molar ratio of 1:1.5:2, then add glacial acetic acid as a solvent, heat up to 80~110°C and stir for 6~12 h, then cool After reaching room temperature, add water into the reactor, then adjust the pH value to 8-10 with 10% sodium hydroxide solution, and filter with suction to obtain a yellow solid. After drying, recrystallize with ethyl acetate, then filter and dry to obtain the intermediate compound ;
[0031] 2. Intermediate compounds Synthesis:
[0032] Weigh 2.0 mmol intermediate compound and 0.35 g of Raney nickel were added to the reactor, then ethanol was added as a solvent, and nitrogen protection was introduced. Under ...
specific Embodiment approach 3
[0035] Specific embodiment three: what this embodiment is different from specific embodiment two is that the acidic medium described in the step 3 is the concentrated sulfuric acid that mass percentage concentration is 98%, the concentrated nitric acid that mass percentage concentration is 65~68%, ice Acetic acid or benzoic acid.
[0036] Others are the same as in the second embodiment.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com