High-purity troxerutin and preparation method thereof
A high-purity, rutin technology, applied in the preparation of sugar derivatives, chemical instruments and methods, sugar derivatives, etc., can solve the problems of large yield loss, high cost, complicated process, etc., to meet quality requirements and production cycle. The effect of shortening and simplifying the separation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
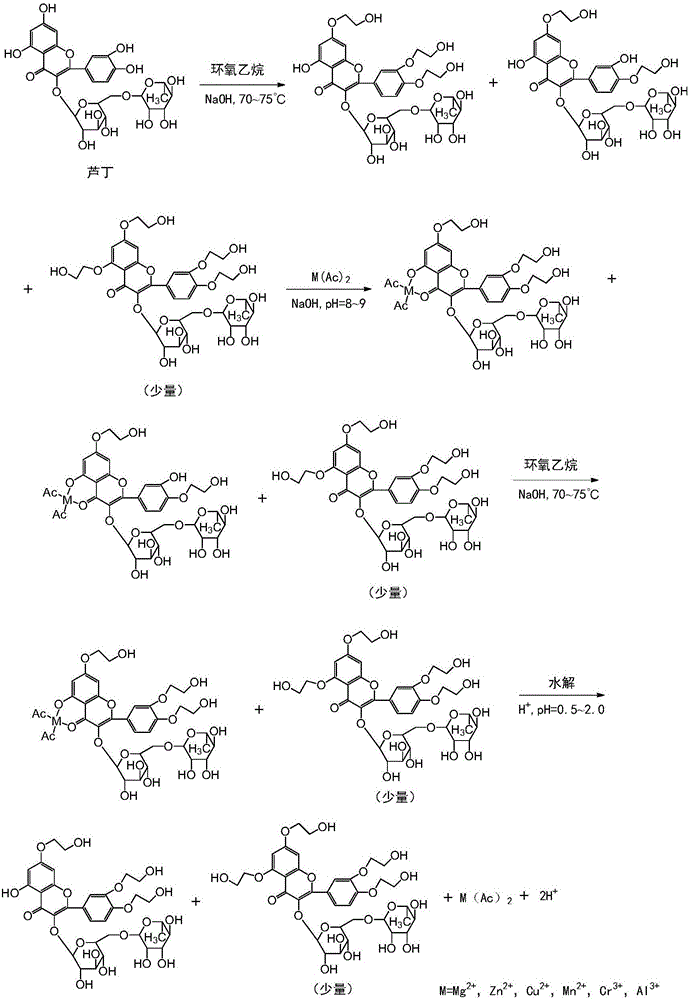
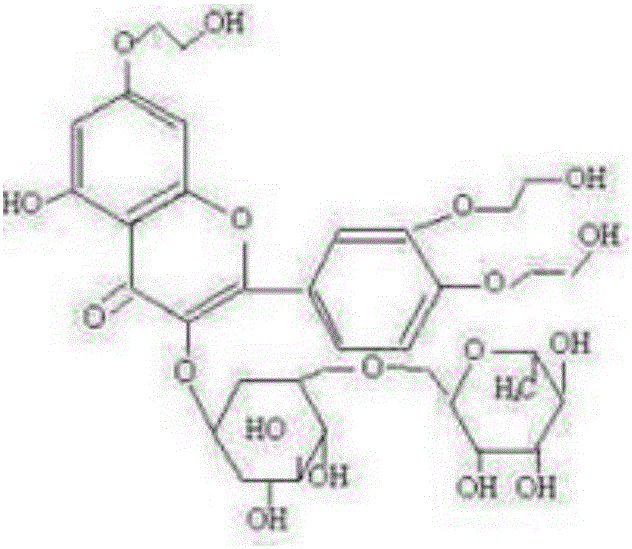
[0027] The preparation method of high-purity troxerutin includes step S1—the first stage etherification step: carry out etherification reaction of rutin and ethylene oxide under the conditions of a mixed solvent system of alcohol and water and an alkaline catalyst, and keep the reaction liquid The peak area percentage of tetrahydroxyethyl rutin in the medium is below 1.5% (detected by high-performance liquid chromatography), and the front-stage etherification reaction is stopped; wherein, rutin, the ethylene oxide, alcohol The mass ratio of water, alkaline catalyst is 1:0.1~0.4:1.5~6.5:0.5~8.0:0.008~0.012, and the reaction temperature of etherification reaction is 60~80°C.
[0028] Wherein, the reaction liquid refers to the reaction liquid during the etherification reaction. The etherification reaction can be carried out according to the conventional etherification reaction operation. Specifically, the etherification reaction is: mix a mixed solvent of alcohol and water with a...
Embodiment 1
[0052] The preparation method of the high-purity troxerutin provided by the present embodiment comprises the following steps:
[0053] S1: The etherification reaction is: mix isopropanol, anhydrous salt water and potassium hydroxide, pass argon into it for 10 minutes, then heat to 60-63°C, add refined rutin with a liquid phase purity of 96.10%, and then pass through Ethylene oxide, and control the reaction pH to 8.0 to 8.3, and the reaction temperature is 60 to 65°C to continue the etherification reaction until the peak area ratio of tetrahydroxyethyl rutin in the reaction liquid is detected by high performance liquid chromatography to be 0.55 % stop ventilation reaction; wherein, the mass ratio of rutin, ethylene oxide, isopropanol, anhydrous salt water, potassium hydroxide is 1:0.32:1.5:2.0:0.008;
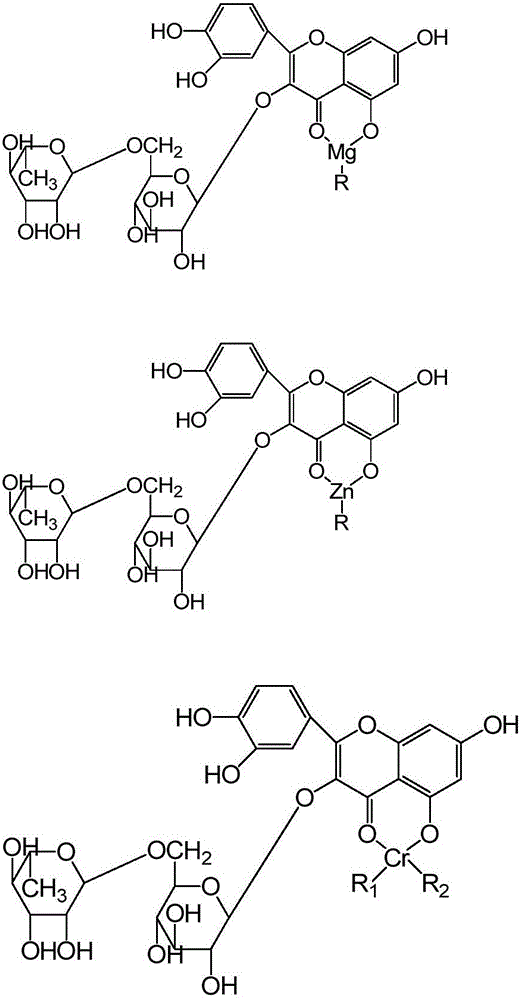
[0054] S2: Adjust the pH of the substance (reaction solution) to 8.0-8.5, and the temperature to 70-75°C, add manganese acetate to the reaction solution, stir for 13 minutes, and...
Embodiment 2
[0061] The preparation method of the high-purity troxerutin provided by the present embodiment comprises the following steps:
[0062] S1: Mix ethanol, ultrapure water and sodium hydroxide, pass nitrogen into it for 12 minutes, then heat to 65°C, add refined rutin with a liquid phase purity of 97%, and then pass in ethylene oxide to control the concentration of the reaction solution. The pH is 8.7-9.0, the reaction temperature is 75-80°C, and the etherification reaction is continued until the reaction liquid is clarified. In the early stage, samples are taken every 1.2 hours for PHLC detection, and the peak area of tetrahydroxyethyl rutin in the reaction liquid is 0.8 % stop the ventilation reaction; wherein, the mass ratio of rutin, ethylene oxide, ethanol, ultrapure water, sodium hydroxide is 1:0.32:2.5:2.5:0.012;
[0063] S2: Adjust the pH of the reaction solution to 9.2 to 9.5 and the temperature to 86 to 90°C. Add a proportioned amount of chromium acetate solution dropw...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com