Neutral and nearly-neutral water oxidation catalytic electrodes in transition metal salt nano arrays
A transition metal salt and nano-array technology, applied in the direction of electrodes, electrode shape/type, electrolysis process, etc., can solve the problems of low catalytic activity, high voltage of hydrogen production system, etc., and achieve the effect of reducing energy consumption and promoting development
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
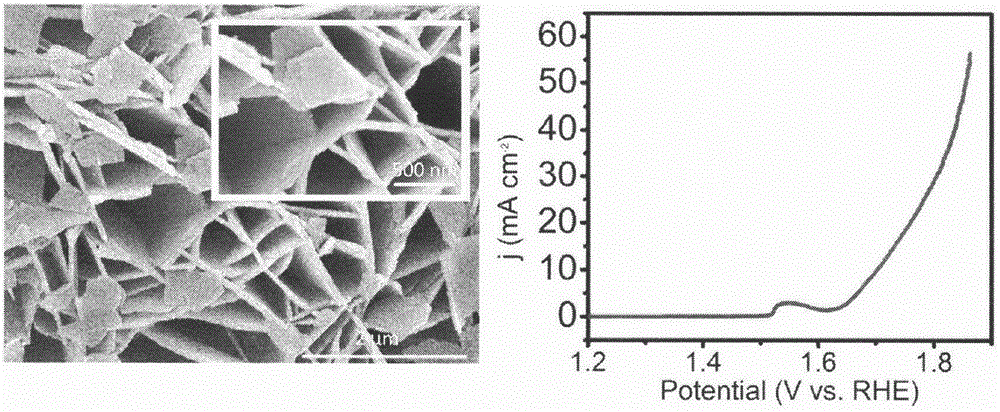
[0020] Step 1: Evenly disperse 1.45g of nickel nitrate and 1.40g of hexamethylenetetramine in 36ml of distilled water, and then transfer the above mixture into a 50ml polytetrafluoroethylene liner.
[0021] Step 2: Put the carbon cloth into the polytetrafluoroethylene lining in step 1, seal the lining with a stainless steel mold, and place it in a constant temperature drying oven at 100°C for 10 hours.
[0022] Step 3: After the reaction, cool down the reactor to room temperature, then take out the carbon cloth and wash it with distilled water and absolute ethanol. Dry in vacuum at 40°C for 24h to obtain Ni(OH) 2 Nanosheet arrays.
[0023] Step 4: Put the precursor prepared in Step 3 in a tube furnace, and react at 300° C. for 2 hours in an argon atmosphere to obtain a NiO nanosheet array.
[0024] Step 5: Use the NiO nanosheet array as the working electrode of the electrochemical workstation, the silver / silver chloride electrode, and the platinum electrode as the reference ...
Embodiment 2
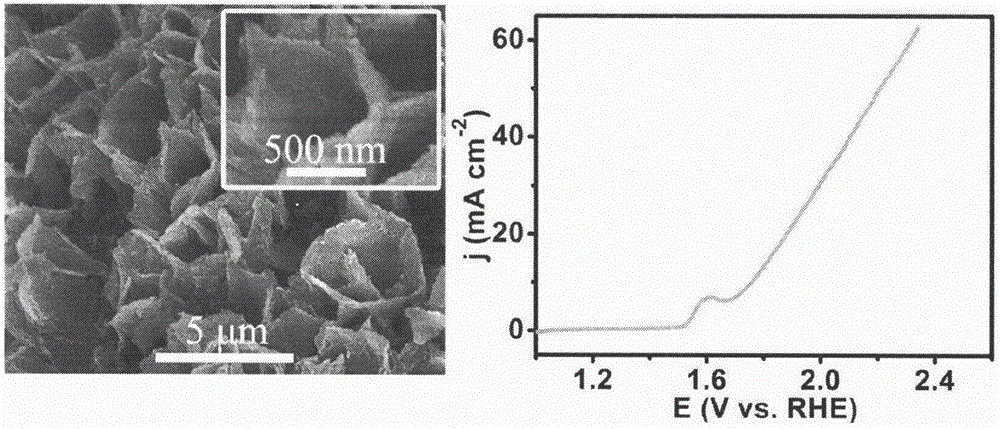
[0027] Step 1: Dissolve 0.87g of cobalt nitrate hexahydrate, 0.29g of ammonium fluoride, and 0.9g of urea in 50ml of distilled water, and then transfer the mixture to 50ml of polytetrafluoroethylene lining.
[0028] Step 2: Put the titanium mesh into the polytetrafluoroethylene lining in step 1, seal the lining into a stainless steel mold, and place it in a constant temperature drying oven at 120° C. for 6 hours.
[0029] Step 3: After the reaction is completed, cool the reaction vessel to room temperature, take out the titanium mesh, wash it, and dry it in a vacuum drying oven at 40° C. for 24 hours.
[0030] Step 4: Place the dried sample in Step 3 and 0.5 g of sodium hydrogen hypophosphite in a tube furnace, and react at 300° C. for 2 hours in an argon atmosphere to obtain a CoP nanowire array.
[0031] Step 5: Use the CoP nanosheet array as the working electrode of the electrochemical workstation, the silver / silver chloride electrode, and the platinum electrode as the refe...
Embodiment 3
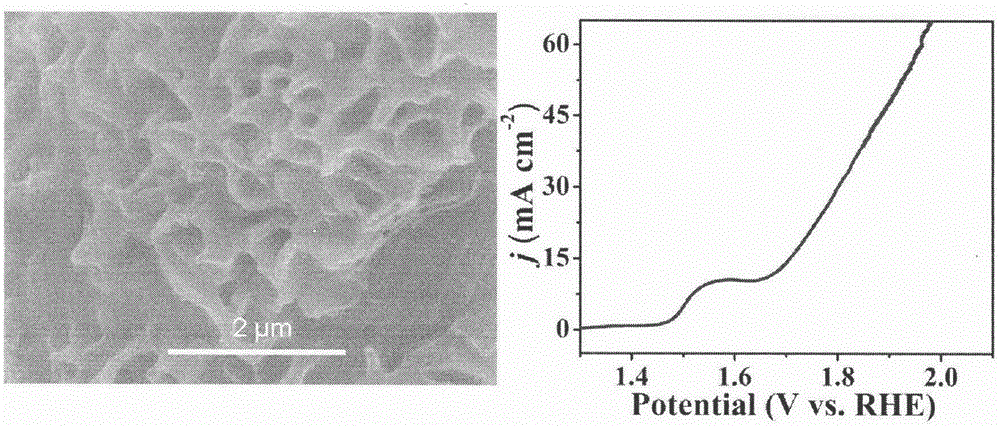
[0034] Step 1: Evenly disperse 1.42g of nickel nitrate and 1.38g of hexamethylenetetramine in 38ml of distilled water, and then transfer the above mixed liquid to a polytetrafluoroethylene liner. Step 2: Put the carbon cloth into the polytetrafluoroethylene lining in step 1, seal the lining with a stainless steel mold, and place it in a constant temperature drying oven at 100°C for 10 hours.
[0035] Step 3: After the reaction, cool down the reactor to room temperature, then take out the carbon cloth and wash it with distilled water and absolute ethanol. Dry in vacuum at 60°C for 24h to obtain Ni(OH) 2 Nanosheet arrays.
[0036] Step 4: Put the precursor prepared in Step 3 and 1g of sodium hypophosphite in a tube furnace, and react at 300°C for 2h in an argon atmosphere to obtain Ni 2 p.
[0037] Step Five: Use Ni 2 The P nanosheet array is used as the working electrode of the electrochemical workstation, and the silver / silver chloride electrode and the platinum electrode ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com