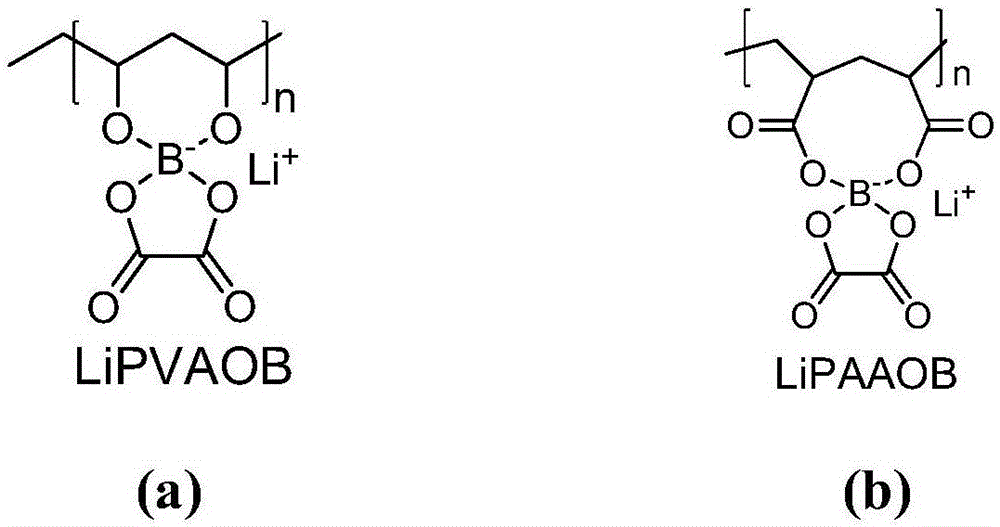Solid polymer electrolyte-based composite gel polymer electrolyte and preparation method and application thereof
A solid polymer and composite gel technology, applied in the field of lithium-ion batteries, can solve the problems of high anion transfer coefficient, high production cost, and difficulty in wide application, and achieve good cycle performance, low production cost, and wide electrochemical window Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] (1) Polyethylene oxide (molecular weight: 100,000), LiClO 4 、TiO 2 (Particle size 50nm) Weigh according to the ratio of mass percentage of 8.7:1:0.3, heat to 120°C under stirring, turn into a uniform solid solution, then cast it on a stainless steel plate, press it to a thickness of 10 microns solid polymer electrolyte membrane and cooled to room temperature.
[0029] (2) In a molten state, a porous polyethylene film with a thickness of 10 micrometers obtained by thermal stretching at 80° C. is placed on the above-mentioned solid polymer electrolyte membrane, and then heated to 60° C. Pressing under 10 atmospheres for 1 hour to obtain a solid polymer electrolyte composite membrane.
[0030] (3) After cutting the above-mentioned composite film into an appropriate size, place it in a vacuum drying oven at 80°C for 24 hours to dry to remove trace moisture, cool it to room temperature in vacuum, and transfer it into an anhydrous and oxygen-free glove box. Then the solid ...
Embodiment 2
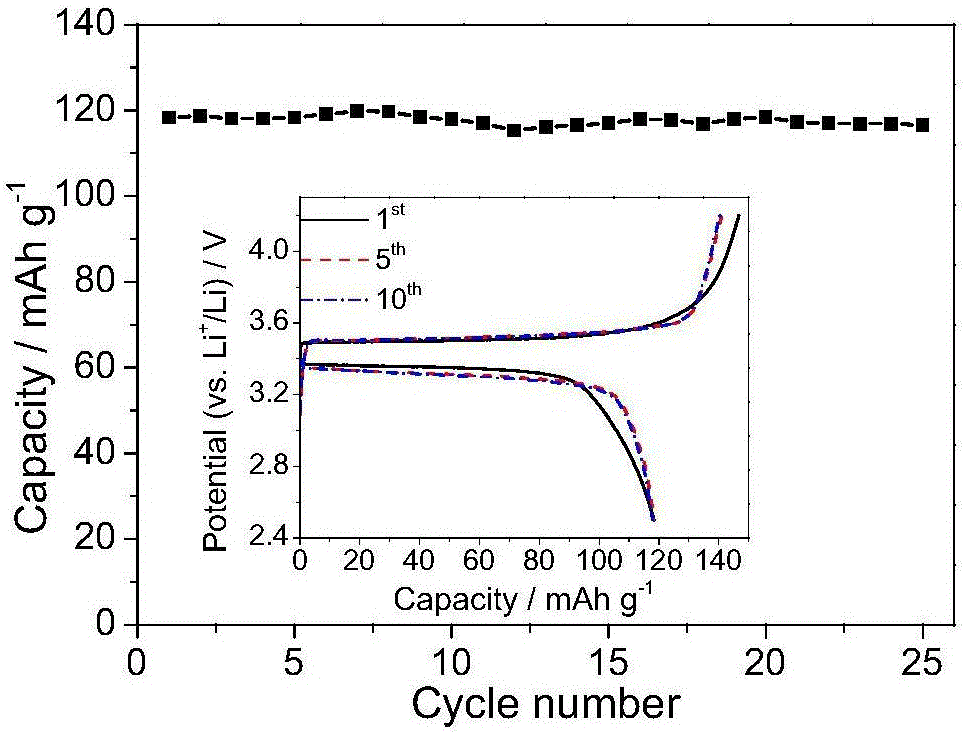
[0043] (1) Dissolve polyvinyl alcohol (PVA, alcoholysis degree ≥ 98%, Mw: 105000, 200mg) and boric acid (148mg) in 20ml dimethylsulfoxide DMSO, then heat at 80°C for 8 hours to obtain a transparent solution. Then add 88mg Li 2 CO 3 And 300mg of oxalic acid, heated to 100 ° C for 24 hours, cooled to room temperature. The solution was poured onto a glass plate, dried in an oven at 70°C, and the solvent was removed to obtain poly(vinyl alcohol-boric acid-lithium oxalate) with a thickness of 30 μm, whose structure is shown in figure 1 a, The ion-conducting group is similar to the structure of LiBOB.
[0044] (2) In the molten state, a porous polypropylene film with a thickness of 10 micrometers obtained by thermal stretching at 100°C, and then the polypropylene film is placed on the above and below the solid polylithium borate film, and then heated to 70°C , pressed at 10 atmospheres for 2 hours to obtain a solid polymer electrolyte composite membrane.
[0045] (3) After cutt...
Embodiment 3
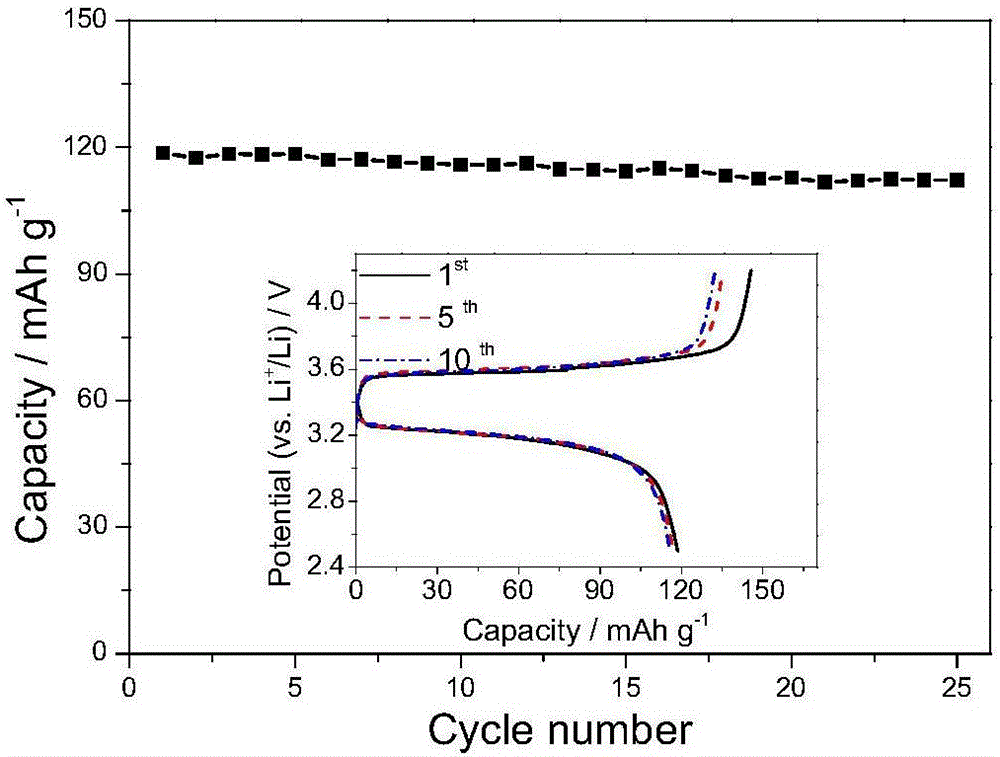
[0048] (1) Polyacrylic acid (PAA, Mw: 450000, 200 g), boric acid (148 g), and 20 ml of deionized water were heated to 80° C. to obtain a transparent solution. Then add 59g LiOH and 300g oxalic acid, heat to 100°C for 24 hours, and cool to room temperature. The solution was poured onto a glass plate, dried in an oven at 70°C, and the solvent was removed to obtain poly(acrylic acid-boric acid-lithium oxalate) with a thickness of 30 μm, whose structure is shown in figure 1 b, The ion-conducting group is similar to the structure of LiBOB.
[0049] (2) P(VDF-HFP) is dissolved in N-methyl-2-pyrrolidone in a ratio of 10% by mass, and then the solution is poured on the poly(acrylic acid-boric acid-lithium oxalate) obtained in the above (1) ) membrane surface, and then heated to 100° C., and vacuum-dried to obtain a solid polymer electrolyte composite membrane.
[0050] (3) After cutting the above composite membrane into an appropriate size, place it in a vacuum drying oven at 80° C....
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com