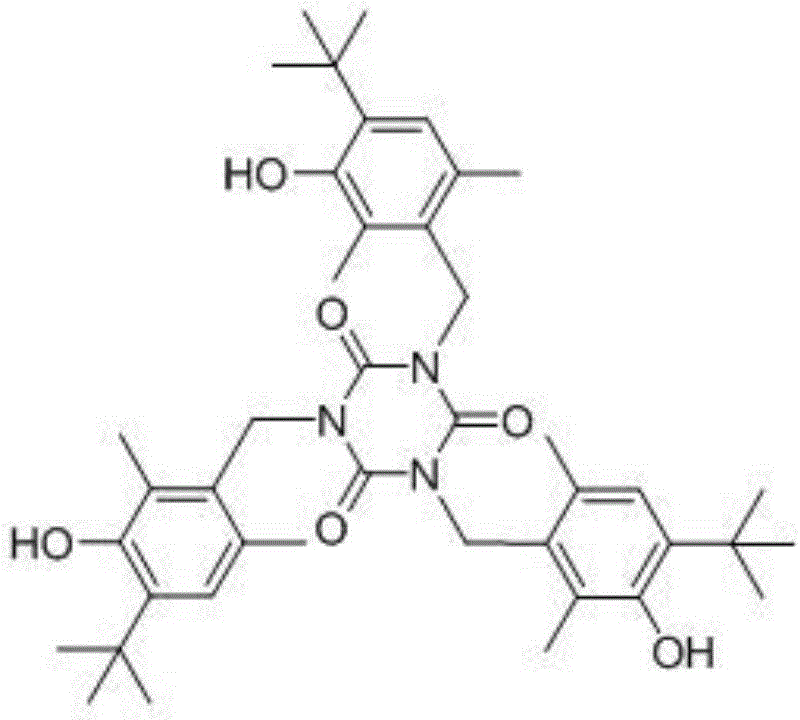
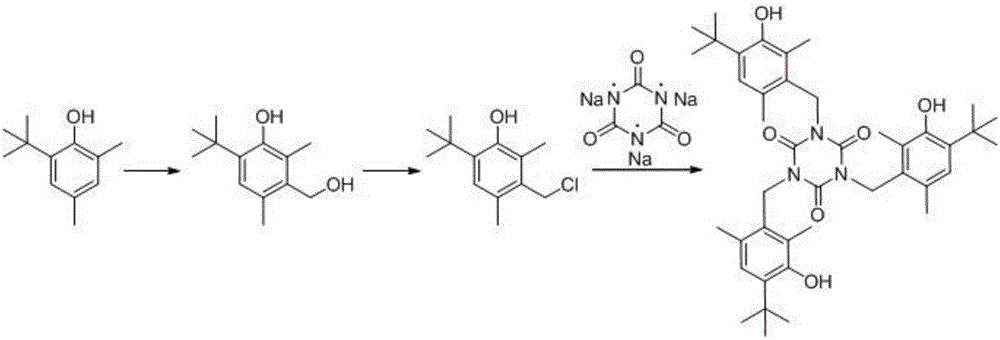
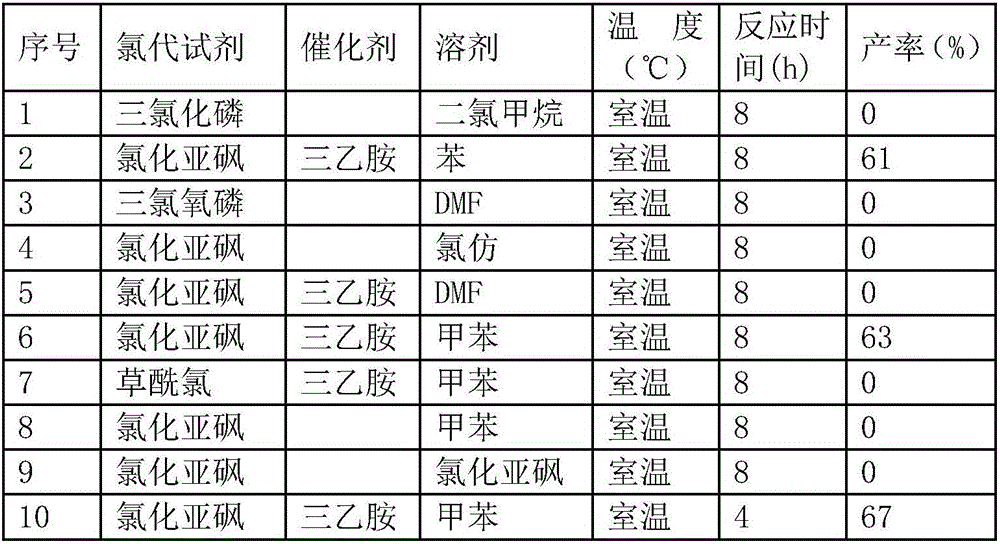
Synthesis method of antioxidant 1790
A synthesis method and antioxidant technology, which is applied in the synthesis field of antioxidant 1790, can solve the problems of high cost, low utilization rate of raw materials, heavy pollution, etc., and achieve the effects of increasing yield, convenient post-processing, and reducing production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] In the three-necked flask, add 2,4-dimethyl-6 tert-butylphenol (1.78g, 0.01mol) and concentrated hydrochloric acid 1.78g, stir, then add paraformaldehyde (0.6g, 0.02mol), and heat up to 38°C , heat preservation reaction for 50 hours, TLC detection raw material still remains. After the reaction, the reaction system was extracted three times with ethyl acetate, the organic layer was collected, dried over anhydrous sodium sulfate, and distilled under reduced pressure to obtain a light yellow oil, the product 4-tert-butyl-3-hydroxy-2,6-dimethyl Benzyl alcohol was directly used in the preparation of 4-tert-butyl-3-hydroxy-2,6-dimethylbenzyl chloride.
Embodiment 2-- Embodiment 4
[0038] Change respectively the temperature described in embodiment 1 and the add-on of concentrated hydrochloric acid, all the other steps and process conditions are identical with embodiment 1, specifically see table 3. As can be seen from Table 3, the result of embodiment 4 is the best, being the best embodiment.
[0039] table 3
[0040] Numbering Concentrated hydrochloric acid (g) temperature(°C) result Example 1 1.78 38 There are surplus raw materials Example 2 1.78 40 There are surplus raw materials Example 3 1.78 42 There are surplus raw materials Example 4 3.56 40 Raw material reaction is complete
[0041] The second big step, the embodiment of preparing intermediate 4-tert-butyl-3-hydroxyl-2,6-dimethylbenzyl chloride:
Embodiment 5
[0043]In the three-necked flask, add 4-tert-butyl-3-hydroxyl-2,6-dimethylbenzyl alcohol (2.08g, 0.01mol), triethylamine (1.21g, 0.012mol) and benzene obtained in Example 4 Thionyl chloride (1.78g, 0.015mol) was added dropwise to 25mL with stirring at 0°C. When the concentration of the raw material was no longer changed by TLC, the reaction was quenched by adding water and extracted three times with ethyl acetate. The organic layer was collected and distilled under reduced pressure to obtain a yellow The oil was dried over anhydrous sodium sulfate, and crystallized with ethyl acetate and petroleum ether as the crystallization solvent to obtain 1.16 g of off-white needle crystals, with a yield of 51%. The proton nuclear magnetic resonance spectrum verified that the substance was the target product 4-tert-butyl-3-hydroxyl-2,6-dimethylbenzyl chloride. Spectral data: 1H NMR (400MHz, CDCl3) δ7.19(s, 1H), 4.73-4.65(m, 2H), 2.59(s, 3H), 2.46(s, 3H), 1.50(s, 9H) .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com