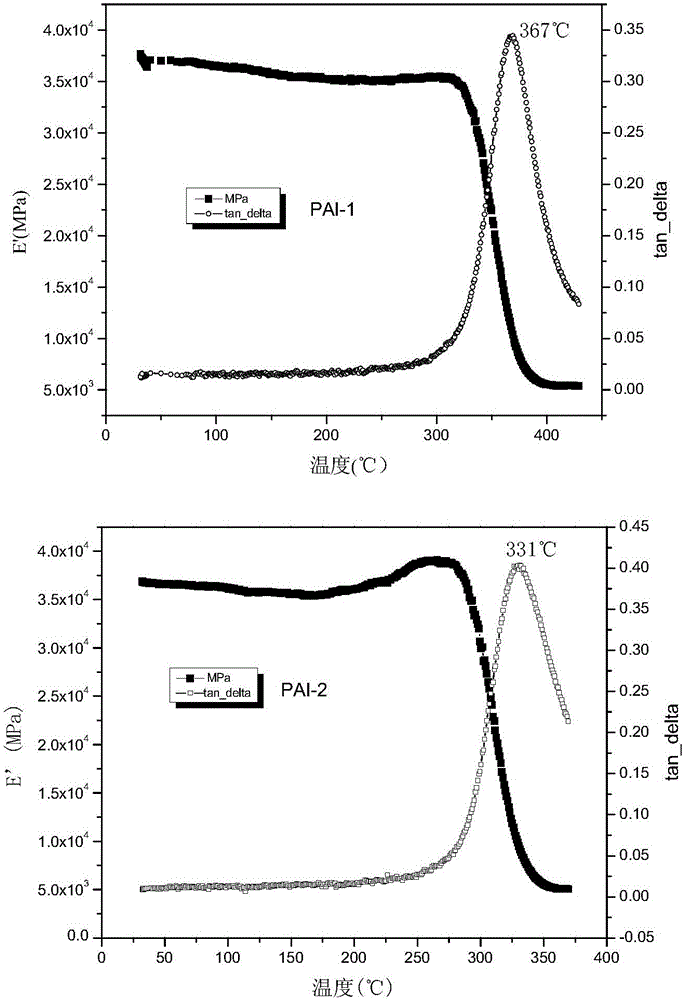
Polyamide-imide with indan structure and preparation method of polyamide-imide
A technology of polyamide and imide, which is applied in the field of polyamideimide resin containing indane structure and its preparation, can solve the problems of difficult processing of large-sized parts and high porosity of parts, and achieve the reduction of bulk density and interaction Function, excellent solubility, and the effect of increasing flexibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] The synthesis of embodiment 1 trimellitic anhydride acid chloride (TMAC)
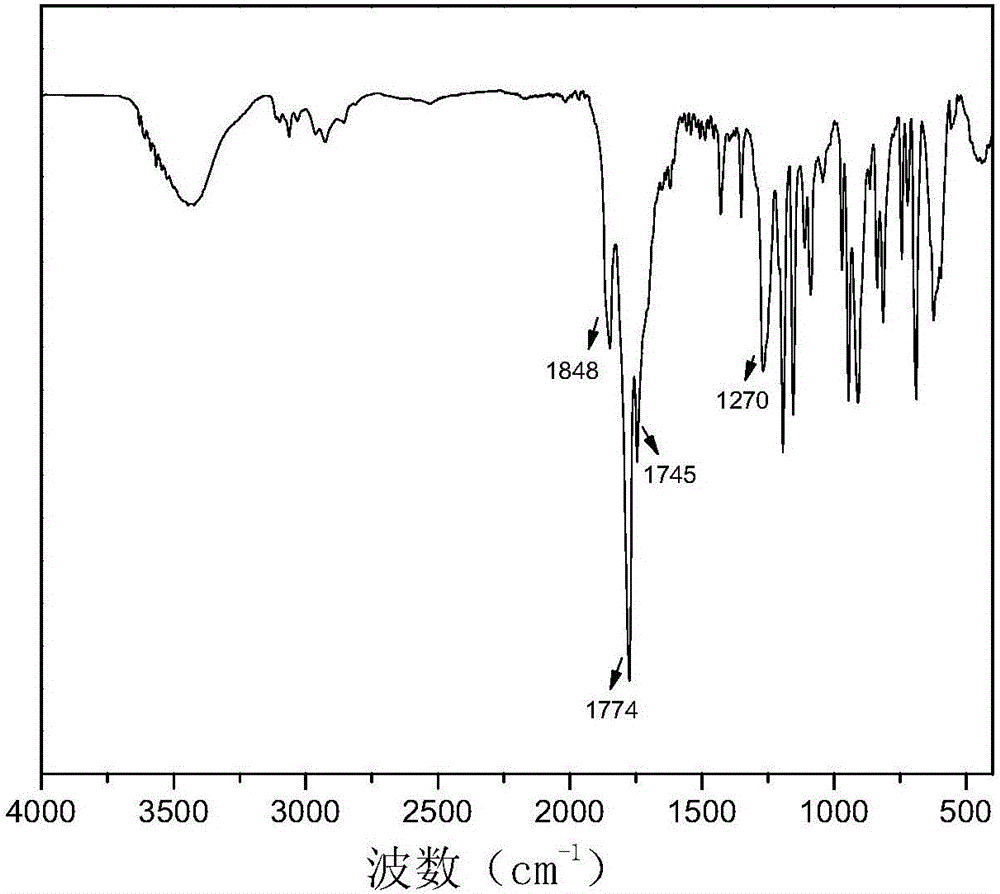
[0049]Add 38g (0.2mol) of trimellitic anhydride and 100ml of thionyl chloride into a three-necked flask equipped with a condensing device and an exhaust gas absorption device, add 1-2 drops of DMF dropwise, heat and reflux at 70°C for 7 hours, and the reaction solution turns into a yellow clear liquid , After distilling off excess thionyl chloride under reduced pressure, a white crystalline solid was obtained after static recrystallization, and a synthetic product was obtained after vacuum drying for 12 hours. m.p.: 66-68°C.
[0050] Infrared analysis of the product see figure 1 , the result of characterization is as follows:
[0051]
Embodiment 2
[0052] Example 2 Synthesis of 5(6)-nitro-1-(4-nitrophenyl)-1,3,3-trimethylindane (PIDN)
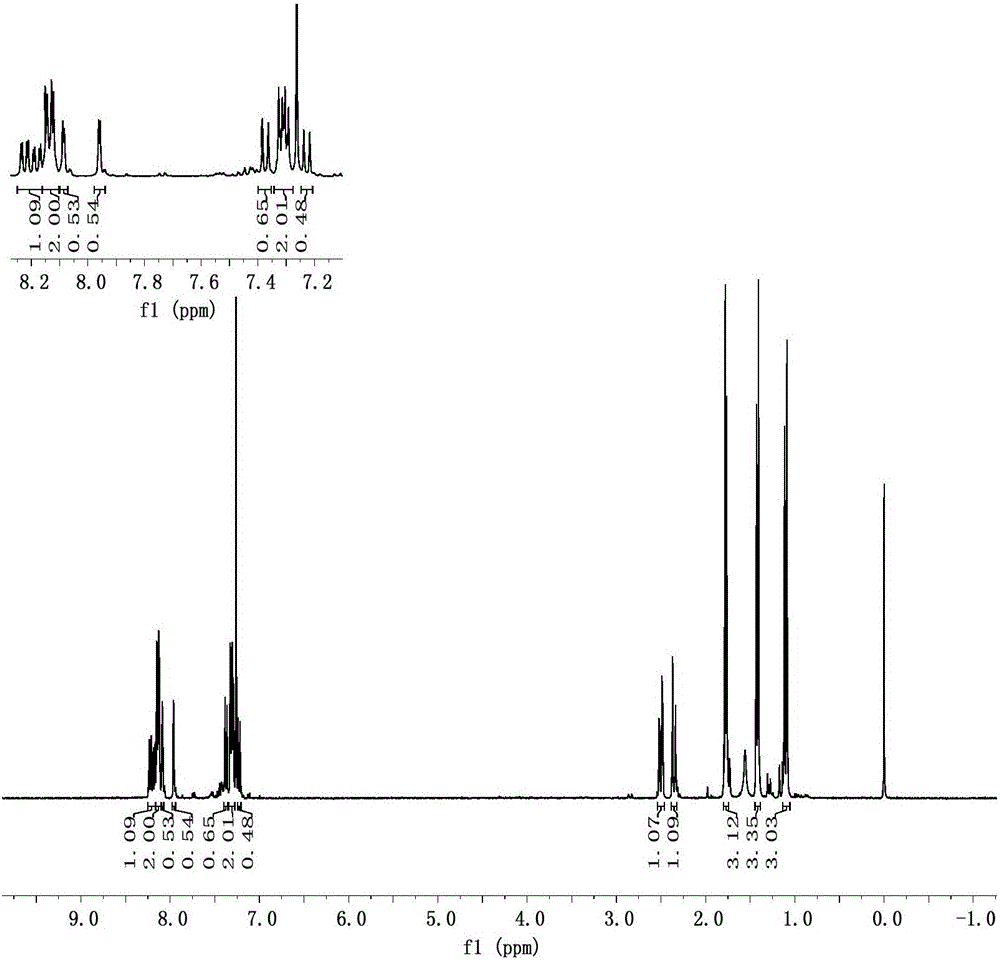
[0053] In a three-necked flask equipped with mechanical stirring, 18.9 g (0.08 mol) of 1,3,3-trimethyl-1-phenylindane was dissolved in 60 ml of dichloromethane, and placed in an ice bath at 0-5 ° C, Slowly add a mixed acid solution with a ratio of 33ml of concentrated sulfuric acid and 14.5ml of concentrated nitric acid dropwise, and determine the end point of the reaction by TCL analysis. After the reaction was completed, the organic layer was separated and kept, washed with saturated sodium carbonate solution to neutrality, then washed with saturated sodium chloride solution, anhydrous MgSO 4 Dry, concentrate under reduced pressure to obtain a brown oily liquid, add petroleum ether, stir at low temperature to precipitate a solid, recrystallize from absolute ethanol, and the yield is 83%. mp: 110-120°C.
[0054] See the NMR spectrum figure 2 , 1 H-NMR (CDCl 3 ,TMS,500MHz), δ:8.25-7...
Embodiment 3
[0055] Example 3 Synthesis of 5(6)-amino-1-(4-aminophenyl)-1,3,3-trimethylindane (PIDA)
[0056] In a 250ml three-necked flask equipped with mechanical stirring and nitrogen gas, dissolve 6g of PIDN in 120ml of absolute ethanol, heat up to 80°C, add 0.3g of Pd / C, slowly add 29ml of 80% hydrazine hydrate solution dropwise, and react for 7 hours Add 0.4g of activated carbon, filter while hot after 1h, concentrate the filtrate under reduced pressure to obtain a brown oily liquid, dissolve in 50ml of 2mol / L dilute hydrochloric acid, stir at room temperature until completely dissolved, extract with ethyl acetate, remove the organic layer, and use Saturated NaOH solution adjusted the pH to alkaline, extracted with ethyl acetate, and concentrated under reduced pressure to obtain a brown oil, which was poured into continuously stirring water to obtain a light brown solid with a yield of 63.7%. mp: 77-98°C.
[0057] 1 H-NMR (CDCl 3 , TMS, 500MHz), δ: 7.13-6.89(m, 3H), 6.70-6.43(m, 4...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com