Prostacyclin Analog Sustained Release Formulation
A technology of sustained-release preparation and prostacycline, which is applied in the directions of drug combination, drug delivery, pill delivery, etc., can solve the problems of unstable physical and chemical properties of the main drug, and achieve the advantages of improving drug compliance, lasting release time and reducing production costs. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
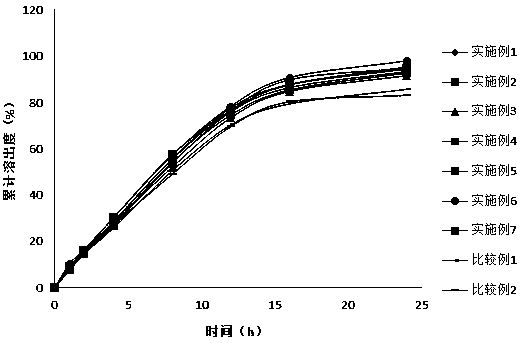
Image
Examples
Embodiment 1
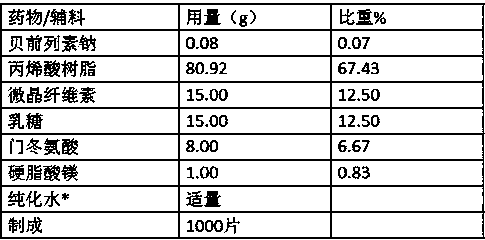
[0030] formula:
[0031]
[0032] Remarks: *Solvent, removed during preparation.
[0033] Preparation:
[0034] (1) Dissolve beraprost sodium and glutamic acid in purified water for later use;
[0035] (2) Put ethyl cellulose and lactose in a wet granulator, use the solution in (1) as a wetting agent for wet granulation, add the wetting agent by spraying, and control the stirring during the granulation process Paddle speed 150~250 r / min, cutter speed 2000~2500 r / min, liquid spray speed 7.5~12.5g / min;
[0036] (3) After the granules are dried, pass through a 30-mesh sieve for sizing, add magnesium stearate for general blending, and compress into tablets.
Embodiment 2
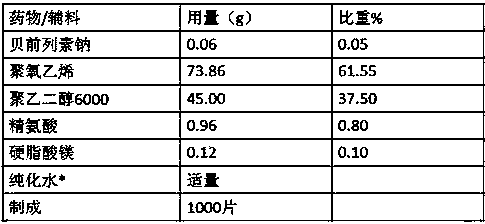
[0038] formula:
[0039]
[0040] Remarks: *Solvent, removed during preparation.
[0041] Preparation:
[0042] (1) Dissolve beraprost sodium and aspartic acid in purified water for later use;
[0043] (2) Put ethyl cellulose, microcrystalline cellulose and lactose in a wet granulator, use the solution in (1) as a wetting agent for wet granulation, add the wetting agent by spraying, and prepare During the granulation process, the speed of the stirring paddle is controlled at 150~250 r / min, the speed of the cutter is at 2000~2500 r / min, and the spraying speed is 7.5~12.5g / min;
[0044] (3) After the granules are dried, pass through a 30-mesh sieve for sizing, add magnesium stearate for general blending, and compress into tablets.
Embodiment 3
[0046] formula:
[0047]
[0048] Preparation:
[0049] (1) Mix Beraprost Sodium, Glycine, Lactose and Glyceryl Behenate evenly by equal volume addition method, and dry granulate with a dry granulator, control the pressure of the pinch wheel at 0.2~0.4MPa, and the speed of the pinch wheel at 30 ~45 r / min, to produce large pieces of particles with suitable hardness;
[0050](2) Pass the above granules through a 30-mesh sieve, weigh them, calculate the amount of magnesium stearate to be added, mix them, and compress them into tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com