Method for CO2 mineralization with blast furnace slag and co-production of aluminum ammonium sulfate
A technology of blast furnace slag and ammonium alum, applied in chemical instruments and methods, inorganic chemistry, cement production, etc., can solve the problem of high energy consumption in mineralization, achieve the effects of reducing environmental pollution, reducing energy consumption, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
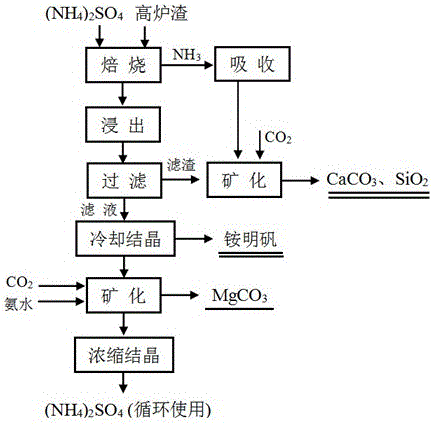
[0023] (1) Blast furnace slag finely ground to below 150μm and ammonium sulfate ((NH 4 ) 2 SO 4 ) are evenly mixed, and the mass ratio of blast furnace slag and ammonium sulfate is controlled to be 1:2.
[0024] (2), put the mixture obtained in step (1) into the tube furnace, ° C / min heat up to 360 ° C and keep warm for 90min to obtain massive solids, and the tail gas produced in the roasting process is absorbed with water to obtain ammoniacal liquor.
[0025] (3), leaching the massive solid obtained in step (2) with water, and controlling the leaching temperature to 90 ° C The reaction time is 10 minutes, and the liquid-solid mass ratio is 3:1. After the reaction is completed, and filtered to obtain the filtrate and filter residue, the filtrate is the crude ammonium aluminum sulfate, wherein the NH 4 Al(SO 4 ) 2 The concentration is about 80g / L.
[0026] (4), the filtrate obtained in step (3) is sent to the crystallization tank, and the temperature of the crystalliz...
Embodiment 2
[0031](1) Titanium-containing blast furnace slag and ammonium sulfate ((NH 4 ) 2 SO 4 ) are evenly mixed, and the mass ratio of blast furnace slag and ammonium sulfate is controlled to be 1:1.
[0032] (2), put the mixture obtained in step (1) into the tube furnace, ° C / min heating up to 250 ° C and keep warm for 240min to obtain massive solids, and the tail gas produced by the roasting process is absorbed by water.
[0033] (3), leaching the massive solid obtained in step (2) with water, and controlling the leaching temperature to be 100 ° C The reaction time is 60 minutes, and the liquid-solid mass ratio is 2:1. After the reaction is completed, and filtered, the filtrate obtained is the thick liquid of aluminum ammonium sulfate, wherein the NH 4 Al(SO 4 ) 2 The concentration is about 140g / L.
[0034] (4), send the filtrate obtained in step (3) into the crystallization tank, and control the temperature of the crystallization tank to be 0 ° C, crystallization for 3 ...
Embodiment 3
[0039] (1) Blast furnace slag finely ground to below 150μm and ammonium sulfate ((NH 4 ) 2 SO 4 ) are evenly mixed, and the mass ratio of blast furnace slag and ammonium sulfate is controlled to be 1:2.
[0040] (2), put the mixture obtained in step (1) into the tube furnace, ° C / min heating up to 450 ° C and keep warm for 30min to obtain massive solids, and the tail gas produced by the roasting process is absorbed by water.
[0041] (3), leaching the massive solid obtained in step (2) with water, and controlling the leaching temperature to be 75 ° C The reaction time is 90 minutes, and the liquid-solid mass ratio is 0.5:1. After the reaction is completed, and filtered, the filtrate obtained is the thick liquid of aluminum ammonium sulfate, wherein the NH 4 Al(SO 4 ) 2 The concentration is about 280g / L.
[0042] (4), the filtrate obtained in step (3) is sent to the crystallization tank, and the temperature of the crystallization tank is controlled to be 5 ° C, cryst...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com