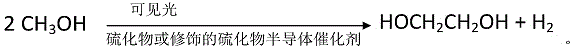Method for preparing ethylene glycol through photocatalytic conversion of methanol
A technology for preparing methanol and ethylene glycol, applied in the field of ethylene glycol, can solve problems such as unreported reports, and achieve the effects of broad application prospects, improved utilization, and low raw material prices
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
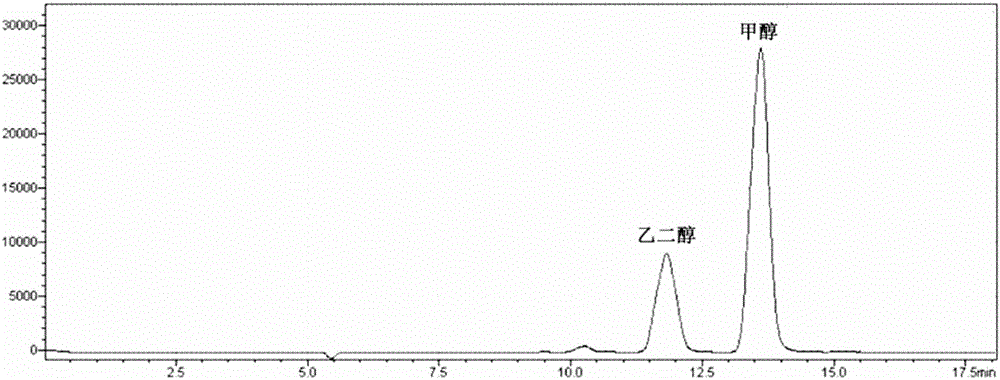
[0041] 5mmol of Cd(NO 3 ) and 5mmol of Na 2 S was added to 80mL water respectively, and stirred thoroughly for 30min. The solution was transferred to a 100mL autoclave, the temperature was raised at a rate of 5°C / min, and the temperature was kept at 180°C for 24h to obtain hydrothermally synthesized CdS nanoparticles. After washing by centrifugation for 3 times, place it in an oven at 60°C for 12 hours, and dry it for later use. 20 mg of the prepared CdS nanoparticles were taken and added to 5 mL of a methanol-water solution with a mass percentage of methanol of 20%. Evacuate or feed inert gas under stirring to remove oxygen in the system, turn on a 300W xenon lamp, and carry out photocatalytic reaction under visible light conditions for 100h. After the reaction solution was filtered, liquid chromatography analysis showed that the conversion rate of methanol was 8.5%, the selectivity of ethylene glycol was 80%, and the yield of ethylene glycol was 6.8%.
Embodiment 2
[0043] Add 5 mmol of Cd(NO 3 ) 2 and 10mmol of (NH 3 ) 2 CS, magnetically stirred evenly, transferred to a 100mL autoclave, heated at a rate of 5°C / min, and kept at 160°C for 48h to obtain CdS nanorods. After washing by centrifugation for 3 times, place it in an oven at 60°C for 12 hours, and dry it for later use. CdS solid powder can be obtained after drying and grinding. 10 mg of the prepared CdS was added to 5 mL of methanol-water solution with a mass percentage of methanol of 80%. Evacuate or pass inert gas under stirring to remove the oxygen in the system, turn on the 500W xenon lamp, and carry out the photocatalytic reaction under the condition of visible light for 90h. After the reaction solution was filtered, liquid chromatography analysis showed that the conversion rate of methanol was 13%, the selectivity of ethylene glycol was 85%, and the yield of ethylene glycol was 11%.
Embodiment 3
[0045] Add 5 mmol of Cd(NO 3 ) 2 and 10mmol of (NH 3 ) 2 CS, magnetically stirred evenly, transferred to a 100ml autoclave, heated at a rate of 5°C / min, and kept at 150°C for 24h to obtain CdS nanorods. After centrifugal washing for 3 times, place in an oven at 60°C for 12 hours, dry and grind to obtain CdS solid powder. Take 0.5 g of the prepared CdS and disperse it into 100 mL of methanol-water solution with a methanol volume fraction of 20%, add 5 μL of chloroplatinic acid solution (0.1 g / ml) according to the loading capacity of 0.1%, stir and pump air. Reduction with a 300W xenon lamp for 1 h yielded CdS loaded with noble metal Pt nanoparticles, ie 0.1% Pt-CdS. Centrifugal washing, drying and grinding for later use. 5 mg of 0.1% Pt-CdS was added to 5 mL of methanol-water solution with a mass percentage of methanol of 95%. Evacuate or pass inert gas under stirring to remove the oxygen in the system, turn on the 300W xenon lamp, and carry out the photocatalytic reactio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com