A water-repellent finishing solution for textiles based on fluorine-containing polyurethane, its preparation method and application
A technology of water-repellent finishing liquid and fluorine-containing alkyl polyurethane, which is applied in the fields of textiles and papermaking, fiber treatment, and liquid-repellent fibers, etc. , Introducing fluoroalkyl and other issues to achieve excellent water repellency, high fluorine content, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
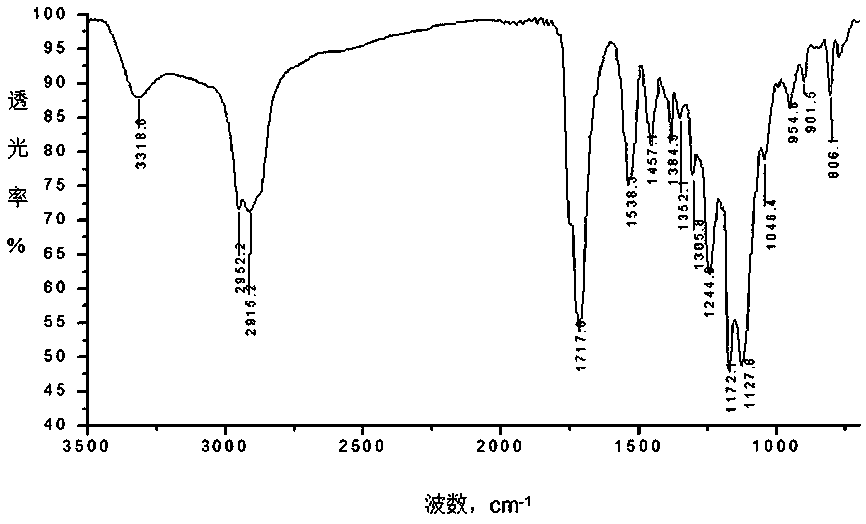
Image
Examples
Embodiment 1
[0034] (1) Synthesis of fluorine-containing alkyl epoxy monomer
[0035] a. Synthesis of nonafluorooxetane
[0036] In a 1000mL three-necked flask equipped with a thermometer, ice-water bath and magnetic stirrer protection, add 16.5g of enbutyl acetate, 26.6g of sodium bicarbonate (NaHCO 3 ), 55.0g sodium dithionite, 220g N,N-dimethylformamide, 210g water, 55.4g perfluorobutyl iodide was added dropwise, reacted at -15°C for 3h, slowly raised to room temperature, and kept for 2h. After the reaction was completed, extraction was performed three times with 150 g of toluene. The extracts were combined, washed 3 times with water to obtain a 4-nonafluorobutyl-3-iodobutyl acetate solution, which was added to a 1000mL three-necked flask, and 305g of a mass concentration was added dropwise to a 20% sodium hydroxide solution. Insulate and react at 15°C for 3h. After the reaction, the layers were separated. The organic layer was washed three times with water, dried and distilled unde...
Embodiment 2
[0058] (1) Synthesis of fluorine-containing alkyl epoxy monomer
[0059] a. Synthesis of nonafluorooxetane
[0060] In a 1000mL three-necked flask equipped with a thermometer, ice-water bath and magnetic stirrer protection, add 16.8g of enbutyl acetate, 27.2g of sodium bicarbonate (NaHCO 3 ), 55.6g sodium dithionite, 225g N,N-dimethylformamide, 200g water, add 55.8g perfluorobutyl iodide dropwise, react at -13°C for 3h, slowly rise to room temperature, and keep warm for 4h. After the reaction was completed, extraction was performed three times with 160 g of toluene. The extracts were combined, washed 3 times with water to obtain a 4-nonafluorobutyl-3-iodobutyl acetate solution, which was added to a 1000mL three-necked flask, and 312g of a mass concentration was added dropwise to a 15% sodium hydroxide solution. Insulate and react at 12°C for 4h. After the reaction, the layers were separated. The organic layer was washed three times with water, dried and distilled under red...
Embodiment 3
[0082] (1) Synthesis of fluorine-containing alkyl epoxy monomer
[0083] a. Tridecafluorooxetane Synthesis
[0084]In a 1000mL three-necked flask equipped with a thermometer, ice-water bath and magnetic stirrer protection, add 16.8g of butyl acetate, 27.9g of sodium bicarbonate, 54.8g of sodium dithionite, 235g of N,N-dimethylformamide, 230g of water, 72.6g of perfluorohexyl iodide was added dropwise, reacted at -14°C for 5h, slowly raised to room temperature, and kept for 6h. After the reaction was finished, extract with 188g trifluorotoluene three times. The extracts were combined, washed 3 times with water to obtain a 4-tridecafluorohexyl-3-iodobutyl acetate solution, which was directly added to a 1000mL three-necked flask, and 511g of a mass concentration of 10% sodium hydroxide solution was added dropwise , kept at 15°C for 6h. After the reaction, the layers were separated. The organic layer was washed three times with water, dried and distilled under reduced pressure...
PUM
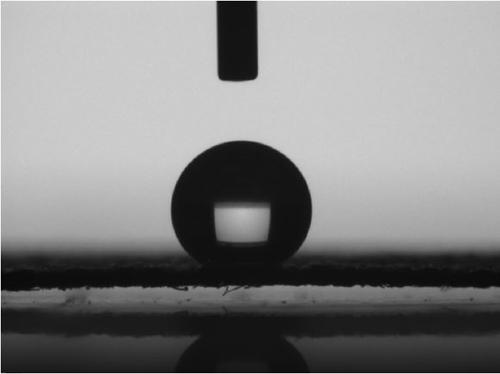
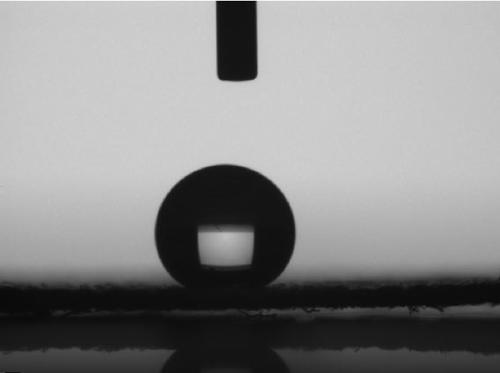
| Property | Measurement | Unit |
|---|---|---|
| contact angle | aaaaa | aaaaa |
| contact angle | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com