High performance liquid chromatography qualitative/quantitative analysis method for amidotrizoic acid active ingredient and application thereof
A high-performance liquid chromatography and quantitative analysis technology, which is applied in the field of drug analysis and detection, can solve the problems of large experimental errors and cumbersome operation of silver nitrate titration, and achieve the effects of ensuring production and quality, solving quality control problems, and ensuring quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
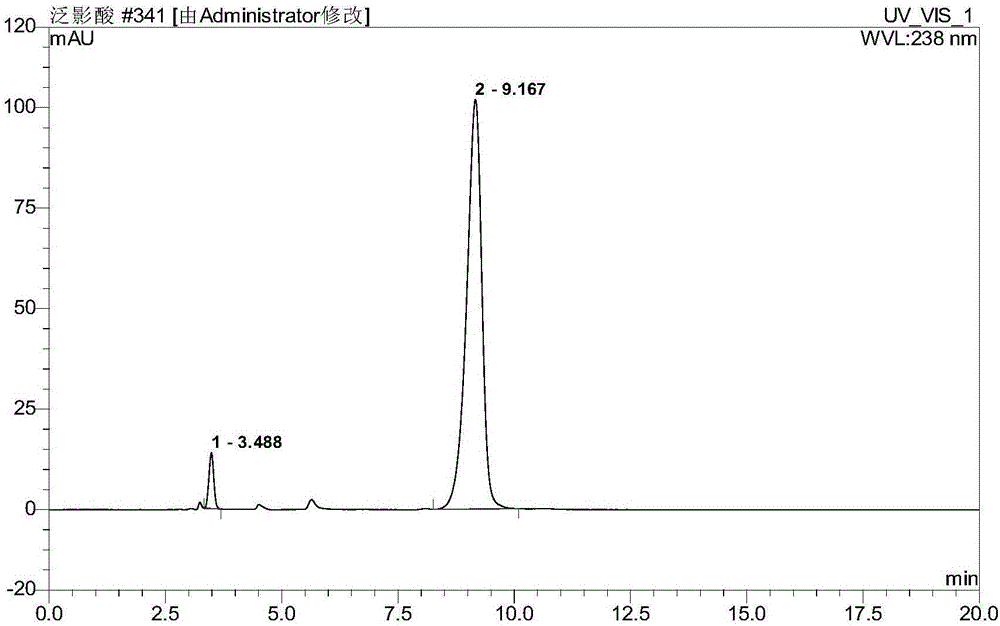
[0044] Embodiment 1 adopts the method of literature to qualitatively detect diatrizoic acid and the raw material nitro substance and key intermediate of synthesizing diatrizoic acid
[0045] In the fifth issue of 2011 of "Chinese Medicine and Clinic", Ning Huiqing and others published a document titled "Determination of Diatrizoate Glumine Injection by High Performance Liquid Chromatography" to detect diatrizoic acid in the preparation. However, there is no description on the intermediates that may exist in the production. For this reason, the applicant uses the methods in the literature to detect the API and the possible related substances in the API, and the steps are as follows:
[0046] (1) Chromatographic conditions
[0047] Mobile phase flow rate: 1.0mL / min;
[0048] Column temperature: 30°C;
[0049] Injection volume: 5μL;
[0050] Detection wavelength: 238nm;
[0051] Chromatographic column: Agilent SB-C18 5μm 150×4.6mm;
[0052] Mobile equipotential elution: met...
Embodiment 2
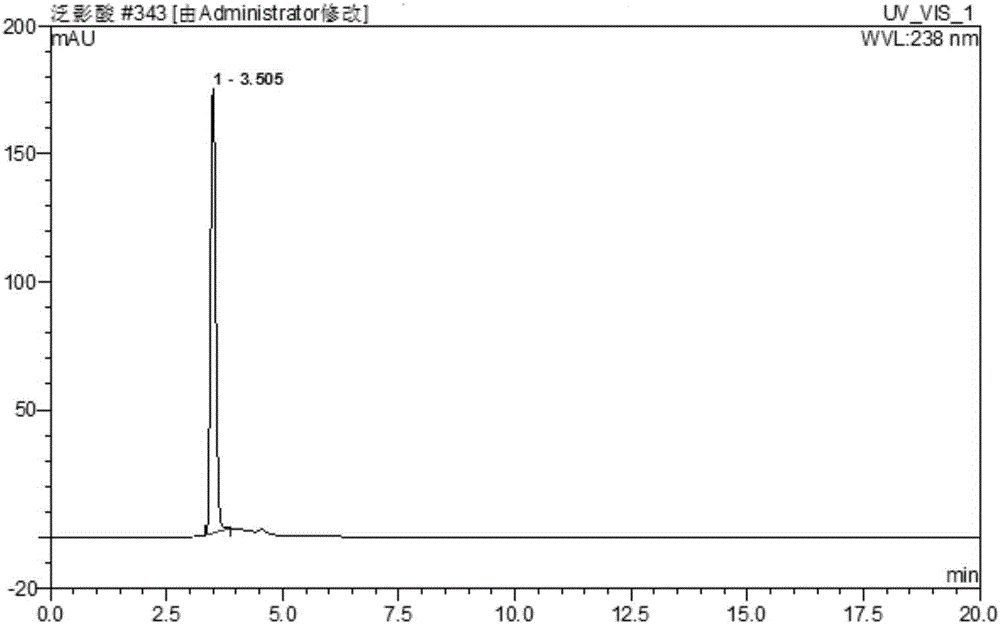
[0058] Embodiment 2 Separation and detection of diatrizoic acid, the raw material nitro substance of synthetic diatrizoic acid, and key intermediates
[0059] (1) Chromatographic conditions
[0060] Mobile phase flow rate: 1.0mL / min;
[0061] Column temperature: 30°C;
[0062] Injection volume: 10μL;
[0063] Detection wavelength: 238nm;
[0064] Chromatographic column: Phenomenex Kinetex 5μm XB-C18 100A 150×4.6mm;
[0065] Mobile equipotential elution: acetonitrile:phosphoric acid water (pH=2.0)=30:70, isocratic elution for 20min.
[0066] (2) Calculation method
[0067] Continuous injection of resolution solution, iodide stock solution, amino stock solution, and nitro stock solution. According to the relative retention time and the ultraviolet absorption spectrum, the related substances that may exist in the raw material drug are qualitatively analyzed through the peak identification of the resolution solution, and the content of impurities in the resolution solution a...
Embodiment 3
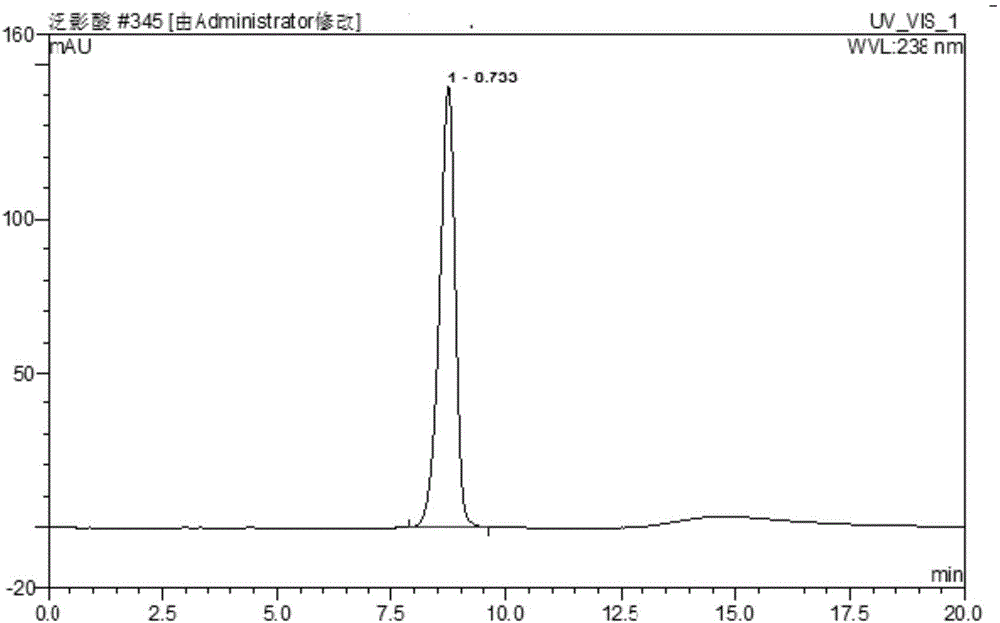
[0071] The content determination of embodiment 3 diatrizoic acid crude drug
[0072] (1) adopt the chromatographic condition identical with embodiment 1, specifically as follows:
[0073] Mobile phase flow rate: 1.0mL / min;
[0074] Column temperature: 30°C;
[0075] Injection volume: 10μL;
[0076] Detection wavelength: 238nm;
[0077] Chromatographic column: Phenomenex Kinetex 5μm XB-C18 100A 150×4.6mm;
[0078] Mobile equipotential elution: acetonitrile:phosphoric acid water (pH=2.0)=30:70, isocratic elution for 20min.
[0079] (2) Calculation method
[0080] Diatrizoic acid content: W 试 =(A 试 m 对 ·W 对 ) / (A 对 m 试 )
[0081] In the formula:
[0082] A 对 - in the reference substance solution, the average value of the peak area of diatrizoic acid;
[0083] A 试 -in the test solution, the average value of the area of diatrizoic acid;
[0084] m 对 - the quality of the diatrizoic acid reference substance, in grams (g);
[0085] m 试 - the quality of diatrizoi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com