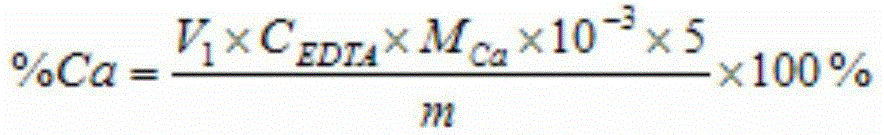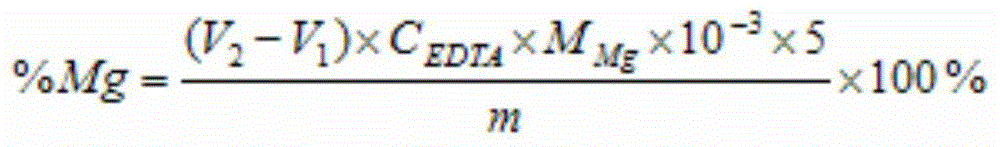Method for rapidly analyzing calcium and magnesium in iron ore
A rapid analysis, iron ore technology, applied in the direction of chemical analysis using titration method, can solve the problems of low measurement results, complicated steps, affecting the accuracy of the results, etc., to ensure the volume of constant volume, the method is simple, fast, and good The effect of practicality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
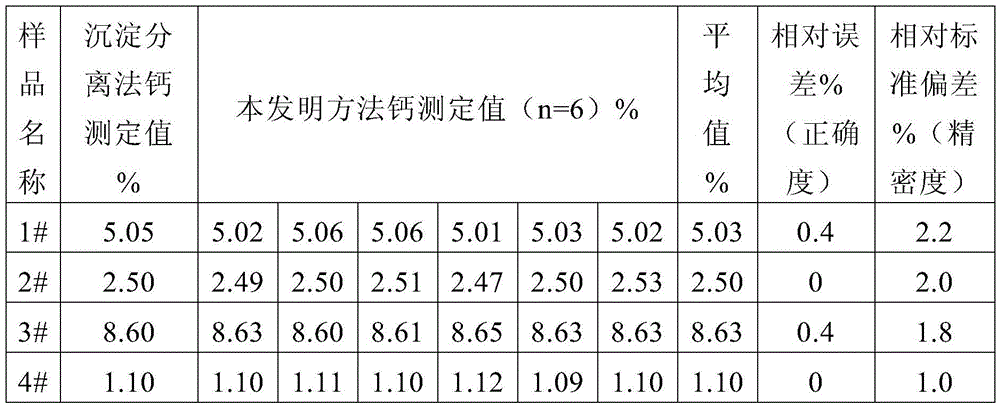
[0037] Weigh 0.5115g iron ore (a certain iron ore in Hubei) sample in a tetrafluoroethylene beaker, after wetting it with water, add 20mL concentrated hydrochloric acid, 10mL concentrated nitric acid, 10mL hydrofluoric acid and 5mL concentrated sulfuric acid to decompose the sample, heat and evaporate When the white smoke is almost exhausted, take it off and cool it, add 5mL6mol / L hydrochloric acid), rinse the cup wall with a small amount of water, heat until the solid is completely dissolved, take it off and cool it, transfer it to a 100mL volumetric flask, and dilute to volume with water. Pipette 20.00mL solution into a glass beaker, dilute to 50mL with water, add 5mL potassium sodium tartrate, 7mL L-cysteine, 10mL potassium hydroxide solution and 0.1g calcium reagent, and titrate with 0.0100mol / L EDTA standard solution to Blue, consume 12.81mL of EDTA standard solution, the calculated calcium content is 5.02%. Then pipette 20.00mL solution into a glass beaker, dilute to 50m...
Embodiment 2
[0039] Weigh 0.5009g iron ore (a certain iron ore in Ethiopia) sample into a tetrafluoroethylene beaker, wet it with water, add 15mL concentrated hydrochloric acid, 10mL concentrated nitric acid, 12mL hydrofluoric acid and 3mL concentrated sulfuric acid to decompose the sample, and heat to evaporate When the white smoke is almost exhausted, take it off and cool it, add 5mL6mol / L hydrochloric acid, rinse the wall of the cup with a small amount of water, heat until the solid is completely dissolved, take it off and cool it, transfer it to a 100mL volumetric flask, and dilute to volume with water. Pipette 20.00mL solution into a glass beaker, dilute to 60mL with water, add 7mL potassium sodium tartrate, 10mL L-cysteine, 10mL potassium hydroxide solution and 0.1g calcium reagent, titrate with 0.0100mol / LEDTA standard solution to blue Color, consumption of EDTA standard solution 6.22mL, the calculated calcium content is 2.49%. Then pipette 20.00mL solution into a glass beaker, dilu...
Embodiment 3
[0041]Weigh 0.4367g iron ore (a certain iron ore in Xinjiang) sample into a tetrafluoroethylene beaker, after wetting it with water, add 18mL concentrated hydrochloric acid, 12mL concentrated nitric acid, 10mL hydrofluoric acid and 5mL concentrated sulfuric acid to decompose the sample, heat and evaporate When the white smoke is almost exhausted, take it off and cool it, add 3mL6mol / L hydrochloric acid), rinse the cup wall with a small amount of water, heat until the solid is completely dissolved, take it off and cool it, transfer it to a 100mL volumetric flask, and dilute to volume with water. Pipette 20.00mL solution into a glass beaker, dilute to 50mL with water, add 6mL potassium sodium tartrate, 8mL L-cysteine, 8mL potassium hydroxide solution and 0.1g calcium reagent, and titrate with 0.0100mol / L EDTA standard solution to Blue, consume 18.81mL of EDTA standard solution, the calculated calcium content is 8.63%. Then pipette 20.00mL solution into a glass beaker, dilute to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com