Preparation method of water-phase cerium fluoride microparticle and application thereof
The technology of phase cerium fluoride and cerium fluoride is applied in the field of preparation of aqueous cerium fluoride particles, which can solve the problems of poor hydrophilicity and complex preparation method, achieve easy operation, simple preparation process, and improve photocatalytic reaction activity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
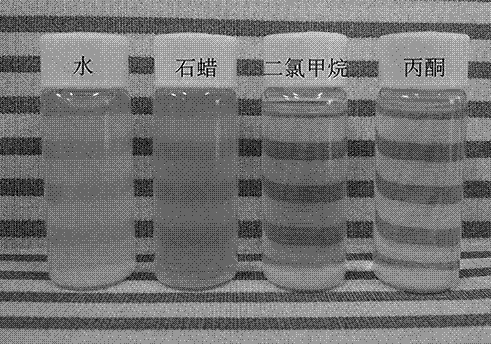
[0034]According to the molar ratio of cerium salt and fluorine-containing ammonium salt of 1:10, take 0.27g of cerium ammonium nitrate and 1.0g of ammonium hexafluorotitanate and dissolve them in 50mL of distilled water, and stir at room temperature for 10~20min to obtain cerium ions and fluoride ions. Transparent solution; stir and slowly add 0.1moL / L ammonium chloride solution to the transparent solution drop by drop until a white sol is formed and stop adding; take the above 30mL white sol and transfer it to a 50mL hydrothermal reaction kettle, and react at 160°C for 300min , cooled to room temperature, and centrifuged to obtain a micropink precipitate; wash the above micropink precipitate with distilled water and absolute ethanol, and dry at 60°C to obtain a cerium fluoride particle sample; disperse the cerium fluoride particle prepared above in water, That is, aqueous phase cerium fluoride fine particles were obtained.
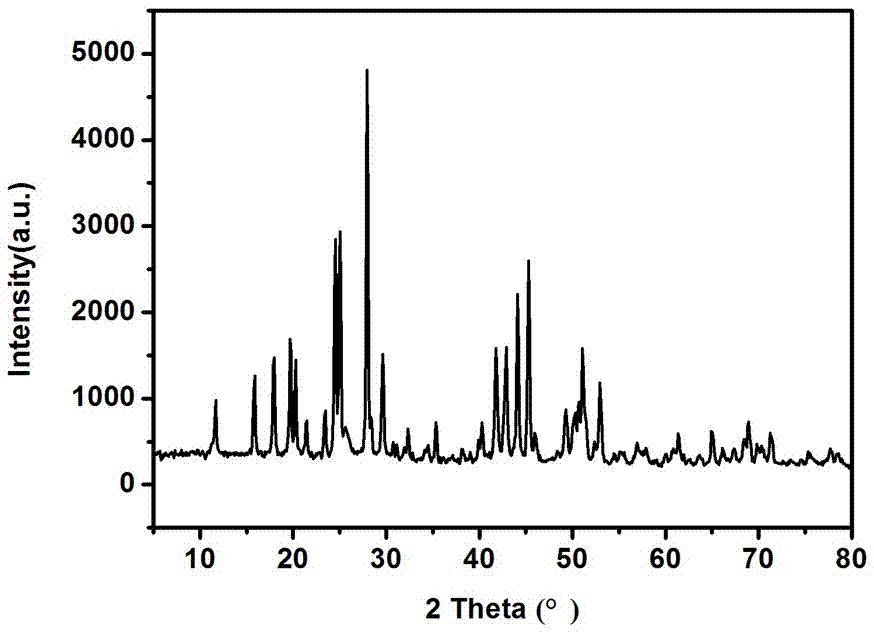
[0035] Such as figure 1 As shown, the XRD diffrac...
Embodiment 2
[0037] According to the molar ratio of cerium salt and fluorine-containing ammonium salt as 1:5, take 0.27g of cerium ammonium nitrate and 0.5g of ammonium hexafluorotitanate dissolved in 50mL of distilled water, and stir at room temperature for 10~20min to obtain cerium ion and fluoride ion. Transparent solution; stir and slowly add 0.1moL / L ammonia water to the transparent solution drop by drop until a white sol is formed and stop adding; take the above 30mL white sol and transfer it to a 50mL hydrothermal reaction kettle, react at 160°C for 300min, and cool to Centrifuge at room temperature to obtain a micropink precipitate; wash the above micropink precipitate with distilled water and absolute ethanol, and dry at 60°C to obtain a sample of cerium fluoride particles; disperse the above-prepared cerium fluoride particles in water to obtain water phase cerium fluoride particles.
[0038] The XRD diffraction pattern of the cerium fluoride particle sample prepared in this examp...
Embodiment 3
[0040] According to the molar ratio of cerium salt and fluorine-containing ammonium salt as 1:7, take 0.43g of cerium nitrate and 1.39g of ammonium hexafluorotitanate and dissolve them in 50mL of distilled water, and stir at room temperature for 10~20min to obtain a transparent solution containing cerium ions and fluoride ions. solution; stir and slowly add 0.1moL / L ammonium chloride solution to the transparent solution drop by drop until a white sol is formed and stop adding; take the above 30mL white sol and transfer it to a 50mL hydrothermal reaction kettle, react at 160°C for 300min, Cool to room temperature and centrifuge to obtain a micropink precipitate; wash the above micropink precipitate with distilled water and absolute ethanol, and dry at 60°C to obtain a sample of cerium fluoride particles; disperse the above prepared cerium fluoride particles in water, that is Aqueous phase cerium fluoride fine particles were obtained.
[0041] The XRD diffraction pattern of the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com