Open framework fluorine-based solid-state electrolyte material and preparation method of open framework fluorine-based solid-state electrolyte material
A technology of solid electrolytes and electrolyte materials, applied in the field of open-frame lithium-rich or sodium-rich fluorine-based solid electrolyte materials and their preparation, can solve the problems of limited electrochemical stability window, instability, complex phase diagram, etc. The effect of improving ionic conductivity, inhibiting battery performance attenuation, and simple process flow
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] 1) Li 3 AlF 6 Ionic Liquid-Based Preparation of Solid Electrolyte Nanoparticles:
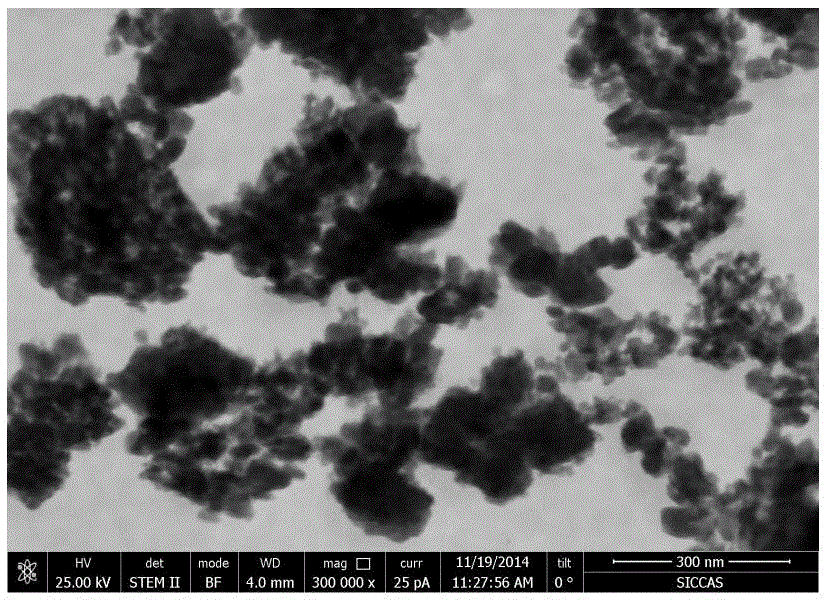
[0055] Weigh 0.3g of Li 2 CO 3 Added to 10 mL of 1-butyl-3-methylimidazolium tetrafluoroborate (BmimBF 4 ) in the ionic liquid, stirred at room temperature for 6 hours to obtain a uniform cloudy solution. Then, while stirring, slowly add 1 g of Al(NO 3 ) 3 9H 2 O, stirring continued for 12 hours to obtain a cloudy solution from which a precipitate could precipitate. The reaction precipitate was repeatedly centrifuged and washed with anhydrous acetone, and vacuum-dried at 80° C. to obtain the solid electrolyte material. Li 3 AlF 6 SEM of solid electrolyte nanoparticles as attached figure 1 Shown, illustrate that embodiment (1) can successfully prepare the nanoscale solid electrolyte material of 20-50nm scale, and because potential surface modification (being the conformal coating of ionic liquid positive group), can reduce particle surface energy, It is conducive to uniform dis...
Embodiment 2
[0057] 1)Na 3 AlF 6 Ionic Liquid-Based Preparation of Solid Electrolytes:
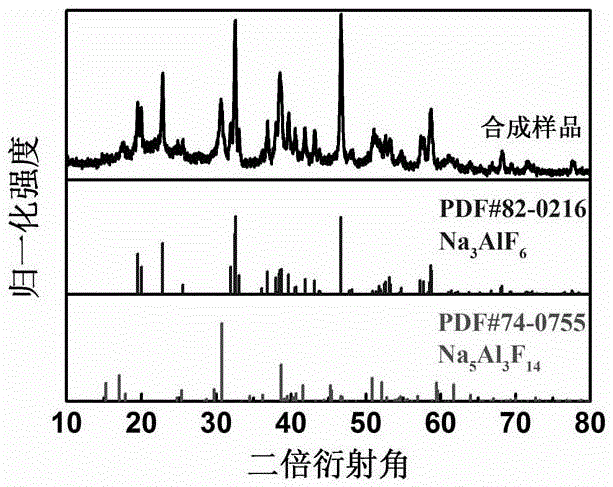
[0058] Weigh 0.424g of Na 2 CO 3 Added to 10 mL of 1-butyl-3-methylimidazolium tetrafluoroborate (BmimBF 4 ) in the ionic liquid, stirred at room temperature for 6 hours to obtain a uniform cloudy solution. Then, while stirring, slowly add 1 g of Al(NO 3 ) 3 9H 2 O, stirring continued for 12 hours to obtain a cloudy solution from which a precipitate could precipitate. Wash the reaction precipitate by repeated centrifugation with anhydrous acetone, and dry it in vacuum at 80°C to obtain Na 3 AlF 6 A solid electrolyte material for the main phase. XRD attached image 3 As shown, a sharper main phase Na can be found 3 AlF 6 The diffraction peak pattern is doped with a broad heterophase Na 5 Al 3 f 14 diffraction peaks.
[0059] 2) Conductivity test of solid electrolyte sheet:
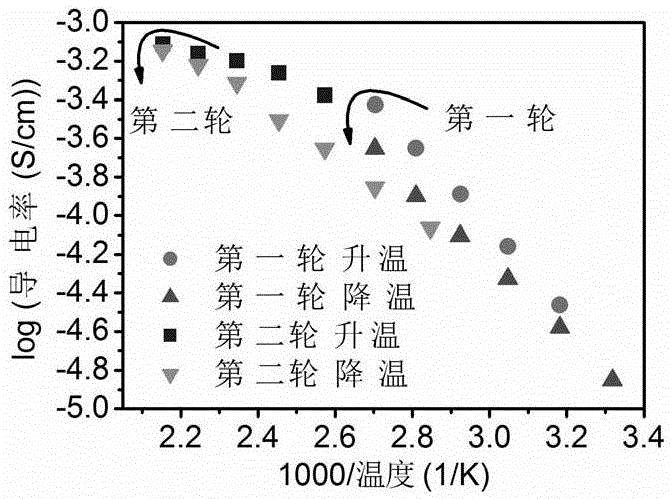
[0060] Na prepared by embodiment 2 3 AlF 6 The solid electrolyte powder is pressed into a disk with a thickness ...
Embodiment 3
[0062] 1)Na 3 Li 3 Al 2 f 12 High-temperature solid-state reaction preparation of solid electrolytes:
[0063] Weigh 0.5g of NaF, 0.31g of LiF and 0.67g of AlF 3 , grind uniformly in the agate grinding body, press the mixture into a disc with a diameter of 10mm under a pressure of 15MPa, and then seal it in a stainless steel reaction kettle filled with an Ar atmosphere, and perform a solid-state reaction at 700°C for 10 hours, and the pressed tablet Crush and grind evenly again to get the desired product. Its XRD is attached Figure 5 As shown, the sharp Na 3 Li 3 Al 2 f 12 Diffraction peaks confirm the phase-pure nature of the material.
[0064] 2) Conductivity test of solid electrolyte sheet:
[0065] Na prepared by embodiment 3 3 Li 3 Al 2 f 12 The solid electrolyte powder is pressed into a disk with a thickness of 1mm and a diameter of 10mm, and the pressure of the tablet is 15MPa. Au electrodes with a diameter of 8mm were vapor-deposited on both sides of t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical conductivity | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com